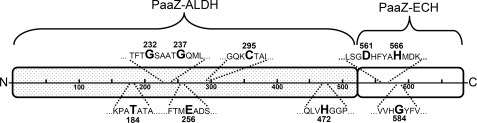
FIGURE 7.
Schematic view of fusion protein PaaZ from E. coli K12. The N-terminal ALDH domain and the C-terminal ECH domain of PaaZ are illustrated as separate parts. The highlighted amino acids are: Thr-184, responsible for cofactor specificity; Gly-232 and Gly-237, important for cofactor binding; Glu-256, likely acting as catalytic general base; Cys-295, catalytic residue responsible for the initial formation of a covalent thiohemiacetal intermediate; His-472, possible candidate for the catalytic general base; Asp-561 and His-566, proposed catalytic residues for hydrolytic ring-cleavage; Gly-584, likely responsible for the formation of an oxyanion hole.