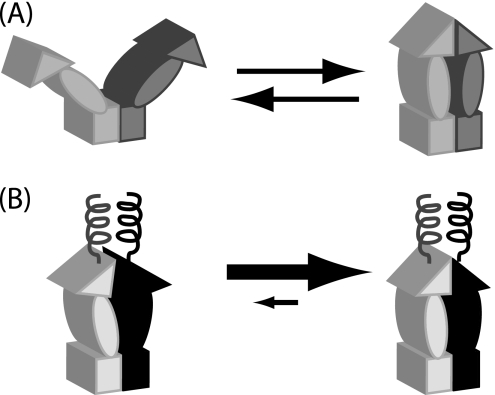
FIGURE 1.
Conformational models of Hsp90 and the influence of engineered N-terminal coiled-coils. A, wild-type Hsp90 contains a stable C-terminal dimerization domain illustrated as a rectangle, a middle domain shown as an oval, and a transient N-terminal dimerization domain illustrated as a triangle. Transient association of the N-domain leads to open and closed states with large conformational rearrangements. To investigate the role of these large conformational rearrangements, we engineered a stable coiled-coil dimerization domain at the N terminus. B, stable association of the N-terminal coil was designed to enforce N-domain proximity, thus increasing N-domain effective concentration and favoring N-domain self-association.