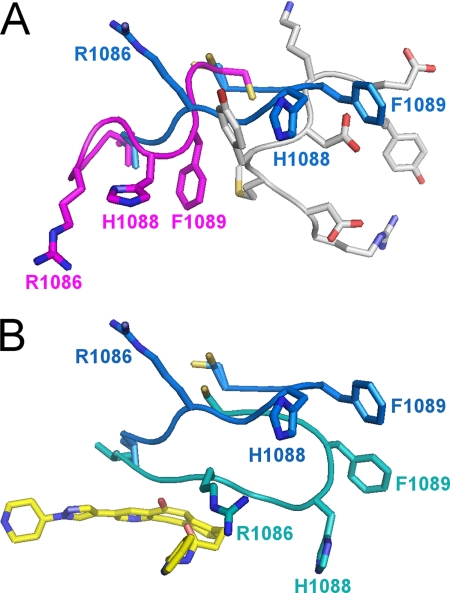
FIGURE 3.
Conformation of the G loop of the c-Met kinase domain in various states. A, in the unphosphorylated state, the G loop (magenta) packs against the activation loop (gray) in a compact conformation that occludes the ATP-binding site. Upon phosphorylation, the G loop adopts an extended conformation (blue) that requires ejection of the activation loop (gray) and reveals the ATP-binding site. The side chains of Arg-1086, His-1088, and Phe-1089 undergo substantial conformational changes (9–11 Å for the Cβ atoms). B, in the ligand-bound state, the G loop undergoes an induced fit conformational change to cap the binding site. The side chains of Arg-1086, His-1088, and Phe-1089 undergo substantial conformational changes (6–20 Å), and His-1088 forms a new hydrogen bond with Asp-1204. The G loop in the unbound and bound states is shown in blue and teal, respectively.