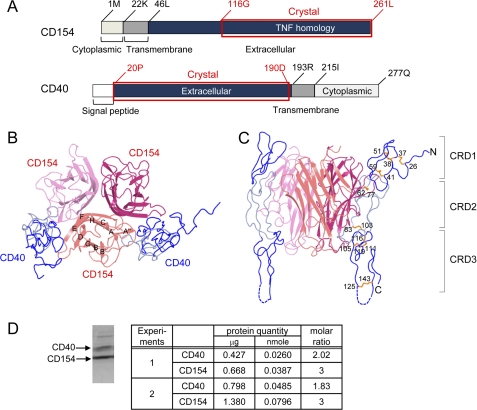
FIGURE 1.
Overall structure of the CD40-CD154 complex. A, domain arrangements of human CD40 and CD154. The crystallized fragments are marked by red boxes and labeled. Top view (B) and side view (C) of the CD40-CD154 complex. The strands of CD154 are labeled. CRD1 and CRD3 of CD40 are colored dark blue, and CRD2 is colored light blue. D, molar ratio of CD40 and CD154 in solution. The purified complex of CD40 and CD154 were separated by SDS-PAGE after partial deglycosylation (see text). The protein bands were stained by Coomassie Brilliant Blue (left), excised, and quantitated by amino acid analysis (right). Samples from two independent preparations labeled as experiments 1 and 2 were analyzed. The minor band in the higher molecular weight region of the SDS-PAGE gel is that of peptide-N4-(N-acetyl-β-glucosaminyl)asparagine amidase added for deglycosylation. It was not subjected to the quantitation analysis. Significant figures of the analysis represent accuracy of the final quantitation step of HPLC chromatograms.