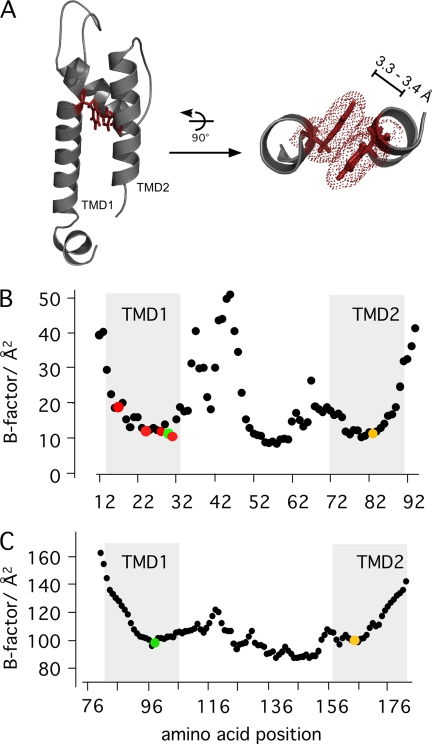
FIGURE 5.
Flexibility of TMDs and aromatic-aromatic interaction between TMD1 and TMD2. A, the distance between Phe-30 in the TMD1 and His-83 in the TMD2 is about 3.3–3.4 Å. This is the typical spacing for π···π interactions (π-stack). Therefore, the two aromatic residues can contribute via this helix-helix interaction to larger protein stability. Cα B-factor distribution of Kcv (B) and in Kir2.2 (C) (4). B, the distribution of B-factors in Kcv as a function of the amino acids. The position of the transmembrane domains is indicated by a gray background. Critical amino acids are highlighted in color: His-83 in yellow and Phe-30 in green. The membrane facing aromatic amino acids His-17, Phe-24, Tyr-28, and Phe-31 are shown in red. C, the B-factors of Kir2.2 (PDB code 3JYC). The position of the interhelical contacts are shown in green (Phe-97) and yellow (Gln-165).