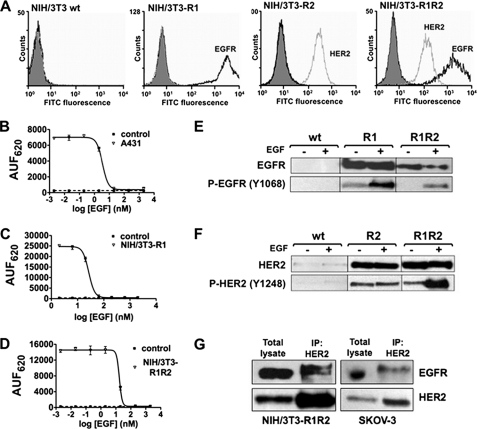
FIGURE 1.
Characterization of the NIH/3T3-HERs cell lines. A, flow cytometry analysis of cell surface EGFR (black) and HER2 (gray) expression using the anti-EGFR antibody m225 and the anti-HER2 antibody FSP77 coupled with a FITC-labeled secondary antibody in parental NIH/3T3 (wt), -R1 (EGFR overexpression), -R2 (HER2 overexpression) and -R1R2 (EGFR and HER2) cells. Controls (gray area) were cells incubated only with the FITC-labeled secondary antibody. B–D, EGF binding in A431 cells (positive control) (B), NIH/3T3-R1 (C), and NIH/3T3-R1R2 (D) cells. Cells were incubated with 4 nm EGF labeled with Lumi4 Tb cryptate and increasing concentrations of unlabeled EGF to compete for binding. Unlabeled EGF induced a dose-dependent inhibition of fluorescence emitted at 620 nm. Data are expressed as arbitrary units of fluorescence at 620 nm (AUF620) and represent the mean ± S.E. of triplicates. E and F, Western blot analysis in parental NIH/3T3 (wt), NIH/3T3-R1, NIH/3T3-R2 and NIH/3T3-R1R2 cells. After stimulation with 100 ng/ml EGF, cells were lysed and protein extracts used to determine the expression and the phosphorylation level of EGFR and HER2 with antibodies against total EGFR (E) or HER2 (F) and against phosphorylated EGFR (E) or HER2 (F). G, presence of EGFR/HER2 dimers in NIH/3T3-R1R2 and SKOV-3 cells. Whole cell lysates from EGF-stimulated cells were immunoprecipitated (IP) using the FSP77 anti-HER2 monoclonal antibody and probed with anti-EGFR and -HER2 polyclonal antibodies to detect HER2 and the amount of co-precipitated EGFR (as a dimerization partner).