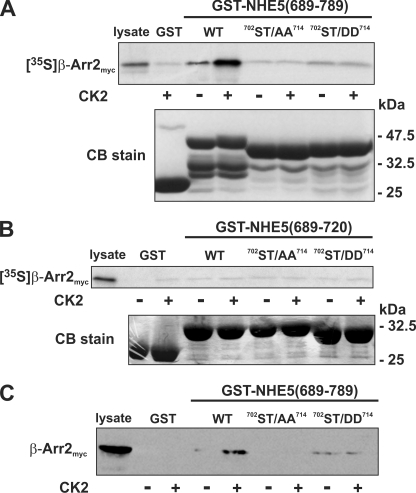
FIGURE 5.
Role of phosphorylation in the in vitro binding of β-arrestin2 to the C terminus of NHE5. A–C, purified WT and mutant (ST/AA and ST/DD) constructs of GST-NHE5(689–789) (A and C) and GST-NHE5(689–720) (B) were preincubated in the absence or presence of purified CK2 and ATP (200 μm). The GST-NHE5 fusion proteins were then incubated in the presence of in vitro synthesized 35S-labeled β-Arr2myc (A and B) or CHO cell lysates containing β-Arr2myc (C). The GST complexes were purified using glutathione-agarose beads and resolved by SDS-PAGE. To measure binding of β-Arr2myc, the gels containing radiolabeled β-Arr2myc were dried, and the signals were detected using a PhosphorImager, whereas those containing non-radioactive β-Arr2myc were subject to immunoblotting. Parallel gels were stained with Coomassie Blue (CB) dye to assess protein loading. Data shown are representative of at least three independent experiments.