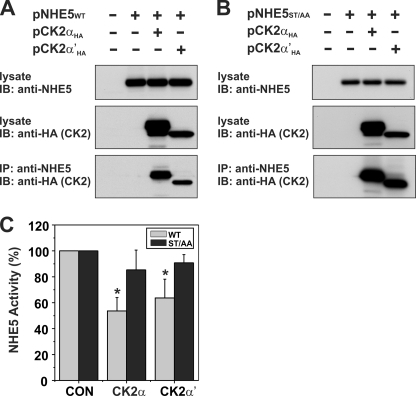
FIGURE 6.
Catalytic α/α′ subunits of protein kinase CK2 form a complex with NHE5 and regulate its activity. Full-length NHE5 WT (A) and mutant (ST/AA) (B) constructs were transiently transfected alone or together with CK2αHA or CK2α′HA in Chinese hamster ovary AP-1 cells. Cells were lysed after 24 h of transfection. Aliquots of the lysates were removed for Western blot analysis of total cellular expression of NHE5 and CK2αHA or CK2α′HA, whereas the remainder was incubated with a rabbit polyclonal anti-NHE5 antibody to precipitate NHE5-containing protein complexes. The immunoprecipitates (IP) were fractionated by SDS-PAGE, followed by immunoblotting (IB) with a monoclonal anti-HA antibody to detect CK2αHA or CK2α′HA. C, AP-1 cells stably expressing NHE5WT or NHE5ST/AA were transiently transfected with CK2αHA or CK2α′HA. Twenty-four h post-transfection, plasmalemmal NHE5 activity was measured as the rate of amiloride-inhibitable, H+-activated 22Na+ influx, as described under “Experimental Procedures.” Values are the means ± S.D. (error bars) of four independent experiments (*, p < 0.05).