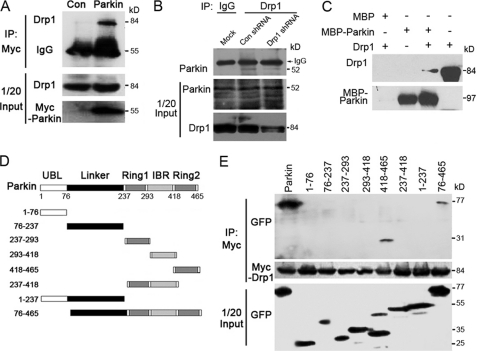
FIGURE 3.
Parkin interacts with Drp1 in vivo and in vitro. A, 293T cells were transfected with Myc-Parkin or the empty vector (control), and immunoprecipitation (IP) and Western blotting were performed to examine the interaction between Myc-Parkin and endogenous Drp1. B, endogenous Parkin (kD) interacts with endogenous Drp1. SH-SY5Y cells were transfected with Drp1 or control shRNAs. Cell lysates were subjected to immunoprecipitation with an anti-Drp1 antibody or an IgG control, and the immunoprecipitates were examined by Western blotting using an anti-Parkin antibody. The middle and bottom panels show the expression of Drp1 and Parkin in the cell lysates (1/20 input). C, Parkin and Drp1 interact in vitro. In vitro translated Drp1 was incubated with bacterially purified MBP-Parkin or MBP immobilized on MBP beads. The presence of Drp1 in the pulldown preparation was examined by Western blotting. The last lane shows 1/20 input of Drp1. D, various truncated forms of GFP-Parkin are shown as a schematic. E, 293T cells were transfected with Myc-Drp1 together with a series of truncated forms of GFP-Parkin. Immunoprecipitation and Western blotting were performed to examine the critical domains in Parkin that mediate its interaction with Myc-Drp1. All data are representative of at least three independent experiments.