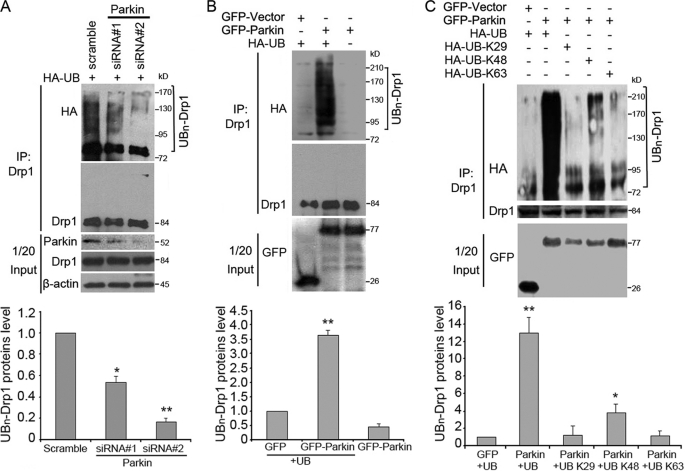
FIGURE 4.
Parkin promotes the ubiquitination of Drp1 in cells. A, SH-SY5Y cells were transfected with HA-UB together with control or Parkin siRNAs as indicated. Cells were treated with MG132 before harvest. Cell lysates were then subjected to immunoprecipitation (IP) and Western blotting to examine the ubiquitination of Drp1. Uniquitinated Drp1 protein level was quantified according to the results of three independent blots. B, 293T cells were transfected with HA-UB together with GFP-Parkin or GFP vector. Cells were treated with MG132 for 8 h before harvest. Cell lysates were then subjected to immunoprecipitation and Western blotting. Uniquitinated Drp1 protein level was quantified according to the results of three independent blots. C, 293T cells were transfected with various HA-UB constructs together with GFP-Parkin or GFP vector. Cells were treated with MG132 for 8 h before harvest. Cell lysates were then subjected to immunoprecipitation and Western blotting. All Western blot data shown in the bar graphs represent means ± S.E. (error bars) from three independent experiments. *, p < 0.01 and **, p < 0.001.