FIGURE 4.
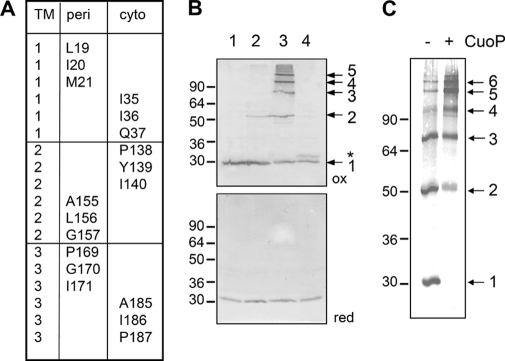
Tandem cysteine scanning of TolQ TMHs. A, summary of the residues present at the cytoplasmic (cyto) and periplasmic (peri) extremities of the TolQ TMHs targeted for the tandem cysteine scanning. All tandem combinations at the cytoplasmic or periplasmic side were tested for their phenotypes (Table 2) and their ability to form disulfide bridges (see supplemental Fig. S4). B, representative profiles obtained for TolQ double cysteine mutants. Membrane extracts from 0.4 × 108 TPS13 (tolQR) cells producing TolR and the WT TolQHA protein (class i, lane 1) or the TolQHA-G157C (class ii, lane 2), -G157C/P169C (class iii, lane 3), or -Q37C/I186C (class iv, lane 4) cysteine mutants were loaded on a 12% SDS-PAGE gel and analyzed by Western blot immunodetections using anti-HA mAb (upper panel, non-reducing conditions (ox); lower panel, β-mercaptoethanol-treated samples (red)). The TolQ monomer and multimers are indicated by arrows on the left. The asterisk indicates a TolQ mobility shift associated with an intramolecular disulfide bond. C, 0.4 × 108 Tuner (DE3) cells producing the TolQ8H-G157C/P169C double mutant, treated (+) or not (−) with the oxidative agent CuoP, were heat-denatured in Laemmli buffer and analyzed by non-reducing 12% acrylamide SDS-PAGE gel and Western blot immunodetection using anti-His5 mAb. Prestained molecular mass markers (in kDa) are indicated on the left.