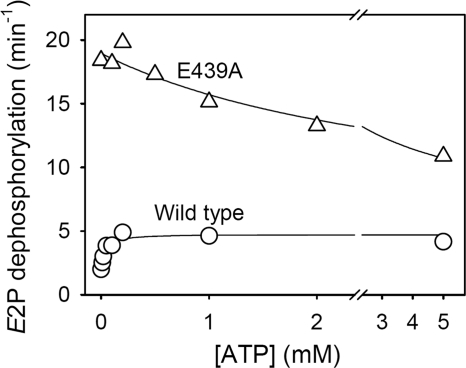
FIGURE 6.
ATP dependence of the rate of dephosphorylation of E2P for mutant E439A. Dephosphorylation of the phosphoenzyme formed in the presence of 32Pi was followed at various ATP concentrations at pH 7.0 in the absence of Mg2+ as described under “Experimental Procedures.” Examples of the decay curves are shown under supplemental Fig. S7. The dephosphorylation rate constants are shown here as a function of the ATP concentration. The parameters derived by fitting a hyperbolic function as described under “Experimental Procedures” are as follows (in each case, the total number of data points included in the fit is indicated in parentheses): wild type, K0.5 = 34 ± 9 μm, k0 = 2.0 min−1, kmax = 4.7 min−1 (n = 22); E439A, K0.5 = 3115 ± 2171 μm, k0 = 19.0 min−1, kmax = 5.6 min−1 (n = 19).