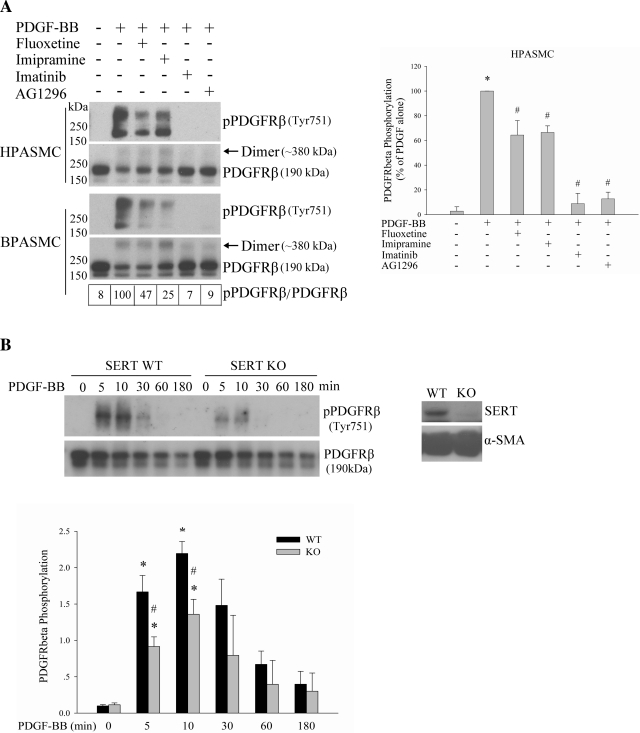
Fig. 5.
SERT partially participates in PDGFRβ phosphorylation by PDGF. A: inhibition of SERT by SERT inhibitors partially blocks PDGF-induced PDGFRβ phosphorylation. Quiescent HPASMCs and BPASMCs were treated with 10 ng/ml PDGF for 10 min with and without pretreatment with 10 μM fluoxetine, 10 μM imipramine, 1 μM imatinib, or 10 μM AG1296 for 30–60 min depending on inhibitors. The phosphorylation of PDGFRβ was analyzed by immunoblotting using a phospho-PDGFRβ (Tyr751) antibody. The relative PDGFRβ activation levels were defined as the ratios of receptor phosphorylation and the receptor levels and expressed as percentage of PDGF alone. Values for BPASMCs are shown in boxes at bottom of top left. Bar graphs (right) represent means ± SD for 3 independent experiments in HPASMCs. *Significant difference from the untreated controls at P < 0.05. #Significant difference from PDGF-treated cells at P < 0.05. B: SERT ablation diminishes PDGFRβ phosphorylation by PDGF. Quiescent SERT−/− and cognate wild-type (WT) rat PASMCs (RPASMCs) were treated with 10 ng/ml PDGF for the indicated time, and phosphorylation of PDGFRβ was determined. The expressions of SERT and α-smooth muscle actin (α-SMA) were detected by Western blotting using SERT and α-SMA antibody, respectively. Quantification of PDGFRβ phosphorylation is shown as means ± SD for n = 3 in bar graph. *Significant difference from the correspondingly untreated SERT−/− and WT cells at P < 0.05. #Significant difference from WT cells treated with PDGF at corresponding time at P < 0.05. “Dimer” noted in Figs. 5 and 6 refers to PDGFRβ, of which the molecular mass is ∼380 kDa. KO, knockout.