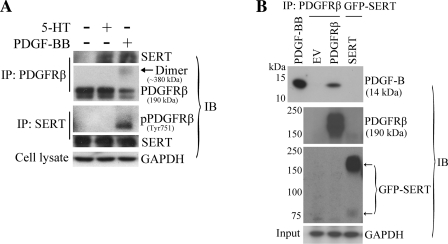
Fig. 6.
Physical interaction between SERT and PDGFRβ. A: PDGF-dependent association of SERT with PDGFRβ in PASMC. Quiescent HPASMCs were stimulated with and without 10 ng/ml PDGF or 1 μM 5-HT for 10 min. A reciprocal coimmunoprecipitation method was employed followed by immunoblotting to detect the physical association of SERT and PDGFRβ with PDGF and 5-HT treatment using SERT and PDGFRβ antibodies. Total GAPDH from whole cell lysates as loading control was detected using the anti-GAPDH antibody. Representative blots are shown. B: PDGF-BB does not bind with SERT directly but rather with PDGFRβ. HEK293 cells were transiently transfected with pcDNA3.1(−) [empty vector (EV)], pEYFP/SERT, pEYFP/SERT-NAE, or pLXSN/PDGFRβ constructs. After 48 h of transfection, cells were lysed and precipitated with green fluorescent protein (GFP) antibody (anti-mouse) and PDGFRβ antibody, respectively. The immunoprecipitates were then incubated with 50 ng of PDGF-BB, and PDGF-BB binding was determined by immunoblotting with a PDGF-B antibody. Pulldown of SERT and PDGFRβ were also determined by GFP antibody (anti-rabbit) and PDGFRβ antibody, respectively. Total GAPDH from whole cell lysates (input) was detected using the anti-GAPDH antibody as loading control. Representative blots are shown. IP, immunoprecipitation; IB, immunoblotting.