Abstract
While anthrax edema toxin produces pronounced tachycardia and lethal toxin depresses left ventricular (LV) ejection fraction in in vivo models, whether these changes reflect direct cardiac effects as opposed to indirect ones related to preload or afterload alterations is unclear. In the present study, the effects of edema toxin and lethal toxin were investigated in a constant pressure isolated perfused rat heart model. Compared with control hearts, edema toxin at doses comparable to or less than a dose that produced an 80% lethality rate (LD80) in vivo in rats (200, 100, and 50 ng/ml) produced rapid increases in heart rate (HR), coronary flow (CF), LV developed pressure (LVDP), dP/dtmax, and rate-pressure product (RPP) that were most pronounced and persisted with the lowest dose (P ≤ 0.003). Edema toxin (50 ng/ml) increased effluent and myocardial cAMP levels (P ≤ 0.002). Compared with dobutamine, edema toxin produced similar myocardial changes, but these occurred more slowly and persisted longer. Increases in HR, CF, and cAMP with edema toxin were inhibited by a monoclonal antibody blocking toxin uptake and by adefovir, which inhibits the toxin's intracellular adenyl cyclase activity (P ≤ 0.05). Lethal toxin at an LD80 dose (50 ng/ml) had no significant effect on heart function but a much higher dose (500 ng/ml) reduced all parameters (P ≤ 0.05). In conclusion, edema toxin produced cAMP-mediated myocardial chronotropic, inotropic, and vasodilatory effects. Vasodilation systemically with edema toxin could contribute to shock during anthrax while masking potential inotropic effects. Although lethal toxin produced myocardial depression, this only occurred at high doses, and its relevance to in vivo findings is unclear.
Keywords: heart function
mortality with shock during the 2001 United States anthrax outbreak was high despite aggressive support (15). The risk of anthrax persists, as evidenced by recent cases of highly fatal soft tissue infections in the United Kingdom (2). Bacillus anthracis produces two toxins: lethal toxin, made up of protective antigen and lethal factor, and edema toxin, made up of protective antigen and edema factor (18, 21, 28, 38). Protective antigen mediates the cellular uptake of the two toxigenic components, lethal and edema factors. Lethal factor is a zinc metalloprotease that inactivates MAPK kinases (18). Edema factor has highly active calmodulin-dependent adenyl cyclase activity that acts to perturb PKA-dependent signaling (21, 25). While lethal toxin and edema toxin are associated with the development of shock during anthrax, the mechanisms underlying these effects remain unclear (28).
One group (36, 37) has shown that rapid edema toxin administration in rats produces hypotension and tachycardia and reduced left ventricular (LV) volumes but not other cardiac parameters. In this and a canine model, lethal toxin decreased LV function (4, 36, 37). This group concluded that edema toxin's recognized effect on tissue edema formation likely reduced preload and induced secondary hypotension and tachycardia. Lethal toxin, on the other hand, appeared capable of depressing myocardial function (9, 36, 37).
We (6, 8, 30) have shown in both rat and canine models that lethal 24-h edema toxin infusions also produce rapid hypotension and tachycardia. In canines, edema toxin reduced central venous pressure and systemic vascular resistance and increased the cardiac index but did not alter the LV ejection fraction (LVEF) (30). The effects of edema toxin in both models persisted after the toxin infusion was stopped. Of note, however, edema toxin was not associated with hemoconcentration, which might have been expected if substantial extravasation of fluid had occurred (6, 30). Based on edema factor's adenyl cyclase activity, these changes appeared potentially related to direct chronotropic or systemic vasodilatory effects. In these same models, lethal toxin produced gradual hypotension not initially associated with tachycardia and progressive (over days) decreases in LVEF (30). Thus, consistent with findings from other laboratories, shock with lethal toxin may be related in part to myocardial dysfunction (9, 22, 36, 37).
It is presently unclear, however, whether edema toxin and lethal toxin alter myocardial function directly or indirectly via their effects on preload or afterload. Therefore, we investigated edema toxin and lethal toxin in a constant-pressure isolated perfused Sprague-Dawley rat heart model. We hypothesized that edema toxin would have chronotropic and potentially inotropic effects via cAMP-mediated mechanisms. We also hypothesized that lethal toxin would depress myocardial function, although it was unclear whether the model would provide sufficient time to measure such changes. Differing doses of each toxin were first assessed. We then investigated the inhibition of edema toxin with either protective antigen-directed monoclonal antibody (PA mAb) or adefovir, an antagonist of edema factor adenyl cyclase activity (27). We also compared edema toxin and dobutamine, an agent with recognized cAMP-mediated myocardial effects (34). Effluent and myocardial cAMP levels were measured during edema toxin perfusion. Finally, we compared the effects of lethal toxin in two strains of rats previously shown to either be sensitive (Sprague-Dawley) or resistant (Lewis) to this toxin's lethal effects (23).
MATERIALS AND METHODS
Animal Care
The protocol used in the present study was approved by the Animal Care and Use Committee of the Clinical Center of the National Institutes of Health.
Study Designs
Constant-pressure nonrecirculating or recirculating isolated Langendorff perfused heart models investigated the effects of edema toxin or lethal toxin on Sprague-Dawley or Lewis rat hearts (male rats, 225–275 g) in the following experiments (Table 1).
Table 1.
Summary of experiments
| Challenge |
Treatment |
||||||
|---|---|---|---|---|---|---|---|
| Experiment | Rat Strain | Method of Perfusion | Agent* | Concentration, ng/ml | Drug# | Dose | No. of Rats |
| 1. Effect of differing edema toxin doses | Sprague-Dawley | Nonrecirculating | Diluent | 0 | 11 | ||
| PA | 100 | 4 | |||||
| PA | 1,000 | 4 | |||||
| ETx | 50 | 5 | |||||
| ETx | 100 | 5 | |||||
| ETx | 200 | 5 | |||||
| 2. Effect of edema toxin inhibition with PA mAb | Sprague-Dawley | Recirculating | PA | 100 | Placebo | 10 | |
| PA | 100 | PA mAb | 10× | 4 | |||
| Diluent | 0 | PA mAb | 10× | 3 | |||
| ETx | 50 | Placebo | 6 | ||||
| ETx | 50 | PA mAb | 10× | 5 | |||
| 3. Effect of edema toxin inhibition with adefovir | Sprague-Dawley | Recirculating | PA | 100 | Diluent | 7 | |
| Diluent | 0 | Adefovir | 0.001 μM | 6 | |||
| Diluent | 0 | Adefovir | 0.01 μM | 3 | |||
| Diluent | 0 | Adefovir | 0.1 μM | 3 | |||
| ETx | 50 | Diluent | 10 | ||||
| ETx | 50 | Adefovir | 0.001 μM | 4 | |||
| ETx | 50 | Adefovir | 0.01 μM | 2 | |||
| ETx | 50 | Adefovir | 0.1 μM | 2 | |||
| 4. Comparison of the effects of edema toxin and dobutamine | Sprague-Dawley | Nonrecirculating | PA | 100 | 5 | ||
| Dobutamine | 12.5 | 6 | |||||
| ETx | 50 | 5 | |||||
| 5. Effect of edema toxin on myocardial and effluent cAMP | Sprague-Dawley | Nonrecirculating | PA | 100 | Placebo | 4 | |
| ETx | 50 | Placebo | 4 | ||||
| ETx | 50 | PA mAb | 10× | 4 | |||
| ETx | 50 | Adefovir | 0.001 μM | 4 | |||
| 6. Effect of differing LeTx doses | Sprague-Dawley | Nonrecirculating | Diluent | 0 | 11 | ||
| PA | 100 | 4 | |||||
| PA | 1,000 | 4 | |||||
| LeTx | 50 | 5 | |||||
| LeTx | 500 | 5 | |||||
| 7. Comparison of the effects of LeTx in Sprague-Dawley versus Lewis rats | Spague-Dawley | Nonrecirculating | PA | 1,000 | 3 | ||
| LeTx | 500 | 3 | |||||
| PA | 5,000 | 3 | |||||
| LeTx | 2,500 | 3 | |||||
| Lewis | Nonrecirculating | PA | 5,000 | 4 | |||
| LeTx | 2,500 | 6 | |||||
ETx, edema toxin; LeTx, lethal toxin; PA, protective antigen;
PA mAb, monoclonal antibody against PA.
Experiment 1: effect of differing edema toxin doses.
Hearts were perfused in a nonrecirculating model with edema toxin at 50, 100, or 200 ng/ml (n = 5 each), diluent alone (n = 11), or protective antigen alone (n = 8). The protective antigen control dose was equivalent to the dose used in the corresponding toxin preparation (see Toxin and Treatments).
Experiment 2: effect of edema toxin inhibition with PA mAb.
To examine whether the cellular uptake of edema factor is necessary for edema toxin's myocardial effects, hearts were perfused in a recirculating model with edema toxin alone (50 ng/ml), edema toxin plus PA mAb (10× the protective antigen molar dose), protective antigen plus PA mAb, or protective antigen alone (n = 6, 5, 7, and 11, respectively).
Experiment 3: effect of edema toxin inhibition with adefovir.
To examine whether cAMP production is necessary for edema toxin's myocardial effects, hearts were perfused in a recirculating model with edema toxin alone (50 ng/ml), edema toxin plus adefovir (0.001, 0.01, or 0.1 μM), adefovir alone, or protective antigen alone (n = 10, 8, 12, and 7, respectively).
Experiment 4: comparison of the effects of edema toxin and dobutamine.
Hearts were perfused in a nonrecirculating model with edema toxin alone (50 ng/ml), dobutamine alone (12.5 ng/ml), or diluent (n = 6, 5, and 5, respectively). This dobutamine dose was determined based on pilot experiments described below.
Experiment 5: effect of edema toxin on myocardial and effluent cAMP levels.
Hearts were perfused in a nonrecirculating model with edema toxin alone (50 ng/ml), edema toxin plus PA mAb, edema toxin plus adefovir (0.001 μM), or protective antigen alone (n = 4 for each group), and effluent and myocardial cAMP levels were measured.
Experiment 6: effect of differing lethal toxin doses on heart function.
Hearts were perfused in a nonrecirculating model with lethal toxin (50 or 500 ng/ml, n = 5 each) or diluent or protective antigen alone (n = 19; same controls as in experiment 1).
Experiment 7: comparison of the effects of lethal toxin in Sprague-Dawley versus Lewis rat hearts.
To examine whether the resistance to the lethal effects of lethal toxin observed in vivo in Lewis rats (23) was reproducible in an isolated heart model, hearts from Sprague-Dawley and Lewis rats were perfused in a nonrecirculating model with lethal toxin at 500 ng/ml (n = 3, Sprague-Dawley rats only) or 2,500 ng/ml (n = 3 and 6 for Sprague-Dawley and Lewis rats, respectively) or protective antigen alone (n = 7).
Langendorff Model
Rats were anesthetized with isoflurane and anticoagulated with heparin (500 IU/kg iv) (29). Hearts were excised rapidly and arrested in cold (4°C) Krebs-Henseleit (KH) buffer with 2 mM sodium pyruvate (14, 29, 35). A cannula was inserted in the aorta, and the heart was perfused retrograde at constant pressure (95 cmH2O) with KH buffer gassed with 95% O2-5% CO2 at 37°C and filtered through a 5-μm filter. As previously described, a water-filled latex balloon connected to a water column and a pressure transducer were introduced into the LV (29). LV end-diastolic pressure (LVEDP) was then adjusted to between 4 and 8 mmHg. Hearts were allowed to equilibrate for 30 min, and baseline readings of LV systolic pressure (LVSP; in mmHg), LVEDP (in mmHg), heart rate (HR; in beats/min), and coronary flow (CF; in ml/min) were then measured (ML880P PowerLab 16/30 with LabChart Pro, AD Instruments, Colorado Springs, CO). If baseline LVSP was ≤80 mmHg, HR was ≤200 beats/min, or CF was ≤ 8ml/min, hearts were excluded.
Nonrecirculating model.
Hearts were perfused with fresh KH buffer over the entire experiment (240 min). Edema toxin, lethal toxin, or dobutamine were infused alone or with treatment through a three-way stopcock at 1/100 the perfusion flow rate starting after equilibration and continuing for 60 min. Hearts were then observed for an additional 180 min except for experiment 5, in which hearts were taken for cAMP measures after 30 min of observation (see below).
Recirculating model.
Hearts were perfused first with fresh KH buffer with 0.2 ml/L antifoaming reagent (FoamAway, Invitrogen, Carlsbad, CA) and then with 300 ml of the same KH buffer premixed with edema toxin alone or in combination with PA mAb or adefovir starting after the equilibration period. The coronary effluent was constantly pumped back into the top reservoir through a 5-μm filter.
Data Collection
Two to four hearts were studied daily, with control and experimental hearts investigated concurrently. Data, including HR, LV developed pressure (LVDP = LVSP − LVEDP; in mmHg), rate-pressure product (RPP = LVDP × HR, in mmHg·beats·min−1), maximal rate of change in LV pressure (dP/dtmax; in mmHg/s), and minimal rate of change in LV pressure (dP/dtmin; in mmHg/s), were recorded continuously. Coronary effluent was collected for CF measurements every 30 min.
Toxin and Treatments
In all experiments except for experiment 7 (comparison of Sprague-Dawley and Lewis rats), toxin components (protective antigen, lethal factor, and edema factor) were recombinant proteins prepared from Escherichia coli as previously described (5, 13, 20). Edema toxin and lethal toxin were composed of edema or lethal factors, respectively, with protective antigen in ratios of 1:2 on the basis of weight (the molecular weights of all three components are very similar). The dose of edema toxin and lethal toxin reported for each experiment reflects the dose of edema or lethal factor used. In toxin dose-response experiments, the initial dose of each toxin investigated (200 ng/ml edema toxin and 50 ng/ml lethal toxin) was equivalent to doses producing an 80% lethality rate (LD80) when administered in the rat in vivo (6). In the experiment comparing the effects of lethal toxin in Sprague-Dawley and Lewis rats (experiment 7), toxin components were recombinant proteins prepared from engineered B. anthracis strains as previously described (21, 24, 26). The lethal factor protein used had the non-native HMAGG NH2-terminal sequence. This preparation of toxin was used in prior experiments investigating the strain sensitivity of rats to the lethal effects of lethal toxin (23). The preparation, when infused over 24 h in the Sprague-Dawley rat, has also been shown to produce hypotension similar to that occurring with lethal toxin prepared from E. coli (6, 8).
The PA mAb (5H3, Human Genomic Sciences, Gaithersburg, MD) used is a human mAb directed against protective antigen. The dose of PA mAb used was 10 times the molar protective antigen dose administered and has been previously shown to improve survival in toxin-challenged rats (7). Adefovir dipivoxil (Sigma Aldrich, St. Louis, MO) was dissolved in ethanol (1 mg/ml) and then diluted to a working solution using KH buffer. In pilot experiments, compared with higher adefovir doses (10 and 1 μM), lower doses (0.1, 0.01, and 0.001 μM) did not alter myocardial function in unchallenged hearts, so these were used in experiments. Both PA mAb and adefovir were mixed along with edema toxin in the 300 ml of KH buffer used in recirculation experiments.
Dobutamine (Bedford Laboratories, Bedford, OH) was diluted in KH buffer. In pilot experiments testing 60-min perfusions with dobutamine in concentrations of 6.25, 12.5, 25, and 50 ng/ml, the two higher doses produced early increases rapidly followed (i.e., within 2–3 min) by decreases in HR and LVDP. In contrast, the two lower doses produced increases in these parameters that persisted during perfusion. Therefore, to compare the effects of dobutamine and edema toxin, a dobutamine dose of 12.5 ng/ml was used.
cAMP and Myocardial Biomarker Measurements
Coronary effluent was collected and frozen on dry ice every 15 min from 0 to 90 min, after which experiments were stopped and heart tissue was frozen in liquid nitrogen. Samples were then immediately assayed for cAMP in triplicate (cAMP Biotrak Competitive Enzyme Immunoassay System, GE Healthcare, Buckinghamshire, UK) by investigators blinded to the study groups. In initial experiments using the recirculating system, cAMP levels were undetectable in both effluent and tissue samples. This was thought potentially to be due to diffusion of excess cAMP out of myocardial tissue and then dilution in the large recirculating volume used. When the experiments were repeated in a nonrecirculating system, cAMP levels were detectable, and these are the results reported. In toxin dose-response experiments, coronary effluent from hearts receiving the highest dose of each toxin tested (i.e., 200 ng/ml edema toxin and 500 ng/ml lethal toxin) were collected at baseline and at 120 and 240 min for measurements of cardiac myosin light chain-1 and troponin using ELISA (Life Diagnostics, West Chester, PA).
Statistical Analysis
Serial changes from baseline were calculated for all parameters except cAMP levels. Continuous data (i.e., HR, LVDP, RPP, dP/dtmax, and dP/dtmin) were averaged over 15-min intervals for each heart. Repeated-measures ANOVA (one or two way, dependent on the comparison) accounting for time and toxin or treatment was performed using PROC MIXED in SAS version 9.2 software (SAS Institute, Cary, NC). In an initial analysis to examine and compare the stability of heart function with the nonrecirculating and recirculating perfusion systems, control hearts (i.e., not perfused with toxin) were analyzed from the toxin dose-response experiments (nonrecirculating) and from the experiments testing PA mAb and adefovir with edema toxin (recirculating). Data for this analysis is shown in Fig. 1 as serial changes from baseline for the respective control groups. In subsequent analyses, serial changes from baseline with toxin or with toxin and treatment were compared with serial changes in controls. However, for clarity in the figures, the serial effects of challenge (i.e., toxin or toxin plus treatment minus the relevant control) are shown. For cAMP data, serial mean data (±SE) are shown. Two-sided P values of ≤0.05 were considered significant.
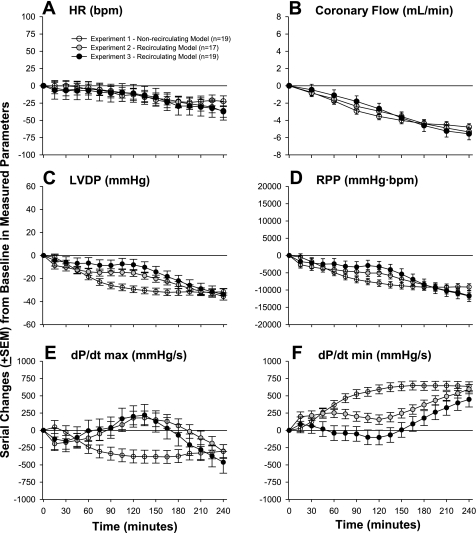
Fig. 1.
Serial changes (means ± SE) from baseline for control hearts in heart rate [HR; in beats/min (bpm); A], coronary flow (in ml/min; B), left ventricular (LV) developed pressure (LVDP; in mmHg; C), rate-pressure product [RPP; in mmHg·beats·min−1 (mmHg·bpm); D], LV dP/dtmax (in mmHg/s; E), and LV dP/dtmin (in mmHg/s; F) from experiments using either nonrecirculating or recirculating perfusion systems. The baseline data that these changes were calculated from are shown in Table 2. The nonrecirculating system was used in the experiment testing the dose responses of edema toxin (ETx), whereas the recirculating system was used for the two experiments testing the inhibition of ETx with either protective antigen (PA)-directed mAb (PA mAb) or adefovir. These controls include hearts perfused with either diluent alone, PA alone, PA mAb without toxin, or adefovir without toxin (see materials and methods and Table 1).
Table 2.
Baseline data for heart rate, coronary flow, LV developed pressure, rate-pressure product, LV dP/dtmax, and LV dP/dtmin in control hearts for the experiments shown in Fig. 1
| Experiment | Heart Rate, beats/min | Coronary Flow, ml/min | LV Developed Pressure, mmHg | Rate-Pressure Product, mmHg·beats·min−1 | dP/dtmax, mmHg/s | dP/dtmin, mmHg/s |
|---|---|---|---|---|---|---|
| 1. Effect of differing edema toxin doses* | 262 ± 5 | 10.1 ± 0.3 | 95.3 ± 2.9 | 24,921 ± 768 | 3,697 ± 80 | −1,816 ± 65 |
| 2. Effect of edema toxin inhibition with PA mAb† | 277 ± 4 | 9.7 ± 0.3 | 86.1 ± 1.7 | 23,906 ± 587 | 2,888 ± 81 | −1,658 ± 43 |
| 3. Effect of edema toxin inhibition with adefovir‡ | 289 ± 9 | 10.0 ± 0.3 | 96.0 ± 4.9 | 26,812 ± 1696 | 3,130 ± 116 | 1,667 ± 67 |
Values are means ± SE.
Controls included animals receiving diluents of edema toxin or PA alone.
Controls included animals receiving toxin diluents with PA-mAb, PA alone, or PA with PA mAb.
Controls included animals receiving toxin diluents with adefovir or PA with diluents of adefovir.
For the toxin dose-response experiments, the effects of diluent alone and protective antigen alone on all cardiac parameters were not significantly different, so these groups were combined as controls. In experiments with PA mAb or adefovir, the effects of protective antigen alone and protective antigen with either PA mAb or adefovir, respectively, on all cardiac parameters were also not significantly different. Therefore, these groups were combined as controls to assess the effects of toxin with or without treatment. Finally, since the influence of the three adefovir doses on the myocardial effects of edema toxin did not differ significantly, they were combined in analysis.
RESULTS
Comparison of Controls from Nonrecirculating and Recirculating Perfusion Models
Figure 1 shows a comparison of controls from the nonrecirculating and recirculating perfusion models. Compared with baseline, control hearts in the nonrecirculating model used for the edema toxin dose-response experiment (see Table 1) demonstrated progressive decreases in HR, CF, LVDP, RPP, and dP/dtmax and increases in dP/dtmin across the 240-min observation period that were significant (P ≤ 0.04). These changes (5–10%/h) were consistent with changes previously reported with this model (29). Control hearts in the recirculating model used for the experiments testing edema toxin inhibition with PA mAb or adefovir (see Table 1) also demonstrated reductions in HR, CF, LVDP, RPP, and dP/dtmax and increases in dP/dtmin by the end of observation. These all occurred in patterns that did not differ significantly in these toxin inhibition experiments except for dP/dtmin (P = 0.03). Overall, however, changes in LVDP, RPP, dP/dtmax, and dP/dtmin occurred more slowly in the recirculating model compared with the nonrecirculating model (P ≤ 0.04).
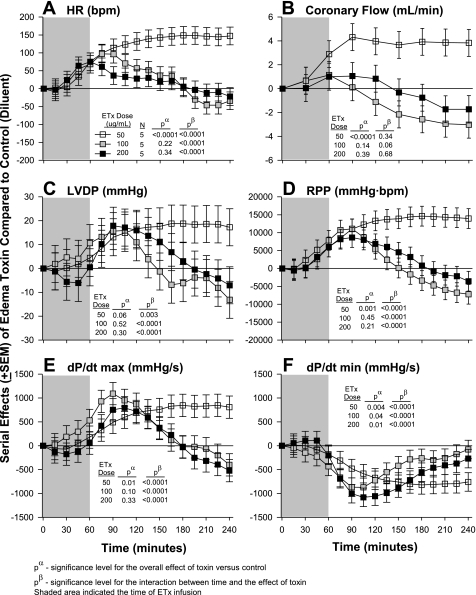
Effects of Differing Edema Toxin Doses on Heart Function
The effects of differing edema toxin doses on heart function are shown in Fig. 2. Compared with controls (see Table 1), the two higher edema toxin doses (100 and 200 ng/ml) produced early increases in HR, CF, LVDP, RPP, and dP/dtmax that then returned to or were less than control levels later in patterns that were significant for all parameters except CF. These two edema toxin doses also produced early decreases in dP/dtmin that returned to control levels later. In contrast, the lowest edema toxin dose (50 ng/ml) produced increases in HR, CF, LVDP, RPP, dP/dtmax and decreases in dP/dtmin that were significant for all except for LVDP (P = 0.06) and became progressively greater over time for all except for CF. Since lethal doses of edema toxin in in vivo experiments caused persistent tachycardia but did not depress LVEF (6, 30), edema toxin at 50 ng/ml was investigated in subsequent experiments. Compared with controls, the highest edema toxin dose (200 ng/ml) did not alter myocardial enzymes levels (myosin light chain-1 and troponin) significantly in the effluent (data not shown), and measures were not made for lower edema toxin doses.
Fig. 2.
Serial effects (means ± SE) of three doses of ETx (50, 100, and 200 ng/ml) compared with controls on HR (A), coronary flow (B), LVDP (C), RPP (D), LV dP/dtmax (E), and LV dP/dtmin (F) in a nonrecirculating model. The shaded area represents when toxin was administered. Levels of significance are shown for the overall effect of toxin with placebo versus control (no toxin) (pα) and the interaction between these effects and time (pβ). As described in materials and methods, for statistical analysis serial changes from baseline with ETx were compared with serial changes from baseline in controls. However, for clarity in A–F, the serial effects of challenge (i.e., toxin minus control) are shown.
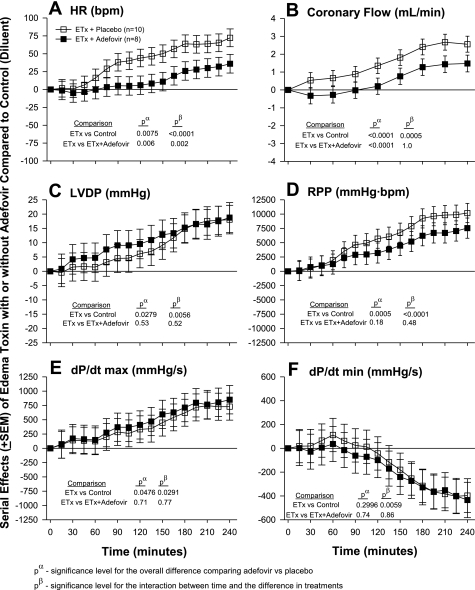
Effects of Edema Toxin Inhibition With Either PA mAb or Adefovir and the Comparison of Edema Toxin and Dobutamine
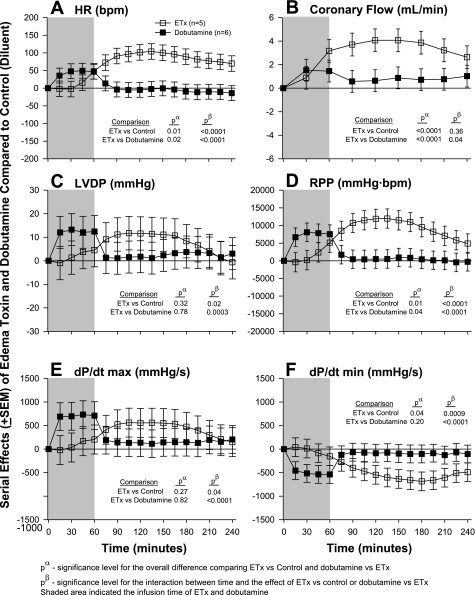
The effects of edema toxin inhibition with PA mAb are shown in Fig. 3 and those with adefovir are shown Fig. 4. The comparison of edema toxin and dobutamine is shown in Fig. 5. Similar to the dose-response experiment noted above, in each experiment shown in Figs. 3–5, compared with control hearts (see Table 1), edema toxin increased HR, CF, LVDP, RPP, and dP/dtmax and decreased dP/dtmin in patterns that were significant (P ≤ 0.05 for the toxin effect or time interaction for all except for LVDP in Fig. 3). Compared with edema toxin alone, PA mAB treatment decreased the effects of edema toxin on HR, CF, RPP, dP/dtmax and increased dP/dtmin in patterns that were only significant for HR and CF (see P values in Fig. 3). Decreases in the effect of edema toxin on HR with PA mAb were greater over time. Adefovir treatment decreased the effect of edema toxin on HR and CF in patterns that were greater over time for the former but not the latter (see Fig. 4 for P values). Compared with controls, dobutamine caused early increases in HR, RPP, dP/dtmax and decreases in dP/dtmin that quickly returned to control levels after the cessation of drug administration (P ≤ 0.02 for the interaction with time). Overall, the effects of edema toxin and dobutamine on all six parameters were very different over time (P ≤ 0.04 comparing the effects of edema toxin vs. dobutamine).
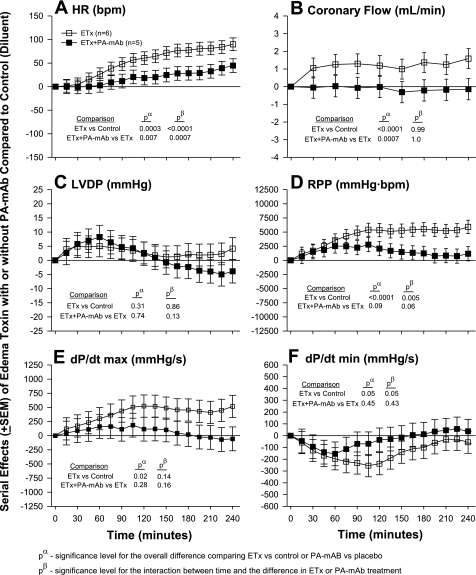
Fig. 3.
Serial effects (means ± SE) of ETx with or without PA mAb compared with controls on HR (A), coronary flow (B), LVDP (C), RPP (D), LV dP/dtmax (E), and LV dP/dtmin (F) in a recirculating model. In this model, hearts were exposed to toxin and treatment throughout the entire perfusion period. Levels of significance are shown for the overall effect of toxin with placebo versus control (no toxin) and versus toxin with PA mAb (pα) and the interaction between these effects and time (pβ). As described in materials and methods, for statistical analysis serial changes from baseline with ETx with or without PA mAb were compared with serial changes from baseline in controls. However, for clarity in A–F, the serial effects of ETx alone or with treatment (i.e., toxin with or without PA mAb minus control) are shown.
Fig. 4.
Serial effects (means ± SE) of ETx with or without adefovir compared with controls on HR (A), coronary flow (B), LVDP (C), RPP (D), LV dP/dtmax (E), and LV dP/dtmin (F) in a recirculating model. In this model, hearts were exposed to toxin and treatment throughout the perfusion period. Levels of significance are shown for the overall effect of toxin with placebo versus either control (no toxin) and versus toxin with adefovir (pα) and the interaction between these effects and time (pβ). As described in materials and methods, for statistical analysis serial changes from baseline with ETx with or without adefovir were compared with serial changes from baseline in controls. However, for clarity in A–F, the serial effects of ETx alone or with treatment (i.e., toxin with or without adefovir minus control) are shown.
Fig. 5.
Serial effects (means ± SE) of ETx (50 ng/ml) or dobutamine (12.5 ng/ml) compared with controls on HR (A), coronary flow (B), LVDP (C), RPP (D), LV dP/dtmax (E), and LV dP/dtmin (F) in a nonrecirculating model. The shaded area represents when toxin or dobutamine was administered. Levels of significance are shown for the overall effect of toxin versus control and toxin versus dobutamine (pα) and the interaction between these effects and time (pβ). As described in materials and methods, for statistical analysis serial changes from baseline with ETx or dobutamine were compared with serial changes from baseline in controls. However, for clarity in A–F, the serial effects of challenge (i.e., ETx or dobutamine minus control) are shown.
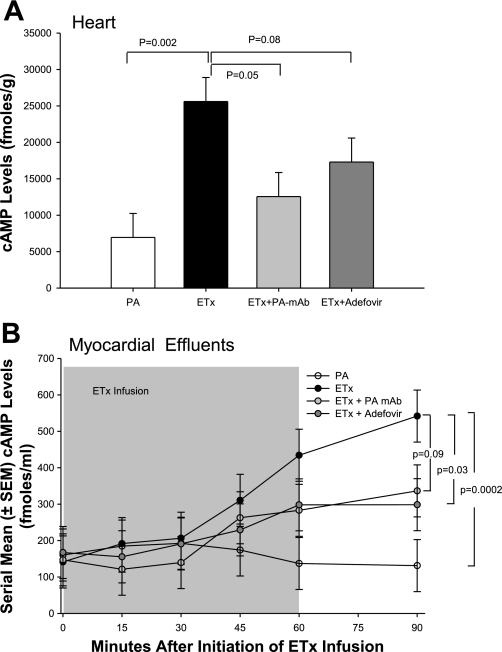
Effects of Edema Toxin Alone or in Combination with PA mAb or Adefovir on Myocardial and Effluent cAMP Levels
Figure 6 shows the effects of edema toxin alone or in combination with PA mAb or adefovir on myocardial and effluent cAMP levels. Compared with control hearts (see Table 1), perfusion with edema toxin alone significantly increased myocardial cAMP levels measured at 30 min after termination of the 60-min edema toxin exposure period. Edema toxin also produced significant increases in cAMP in the effluent that were greater over time after the cessation of edema toxin. Treatment with PA mAb or adefovir in edema toxin-perfused hearts produced decreases in cAMP that were significant for the former but not the latter. These treatments also reduced effluent cAMP levels in patterns that were significant for the latter but not the former.
Fig. 6.
A: cAMP levels (means ± SE) in myocardial tissue 30 min after the completion of 60 min of perfusion with PA alone, ETx alone, or ETx with PA mAb or adefovir. B: serial cAMP levels (means ± SE) in the effluent from hearts perfused for 60 min with PA alone, ETx alone, or ETx with PA mAb or adefovir. The shaded area denotes the time of toxin infusion.
Effects of Differing Lethal Toxin Doses on Heart Function
Figure 7 shows the effects of differing lethal toxin doses on heart function. Compared with controls (see Table 1), the initial dose of lethal toxin tested (50 ng/ml) did not alter any parameter of heart function significantly. In contrast, a higher dose of lethal toxin (500 ng/ml) significantly decreased HR, CF, LVDP, RPP, and dP/dtmax and increased dP/dtmin in patterns that increased over time for all parameters except CF. Compared with controls, the high lethal toxin dose did not significantly alter myocardial enzyme levels (myosin light chain-1 and troponin) in the myocardial effluent (data not shown), and measures were not made for the low lethal toxin dose.
Fig. 7.
Serial effects (means ± SE) of two doses of lethal toxin (lethal toxin; 50 and 500 ng/ml) compared with controls on HR (A), coronary flow (B), LVDP (C), RPP (D), and LV dP/dtmax (E), and LV dP/dtmin (F) in a nonrecirculating model. The shaded area represents when the toxin was administered. Levels of significance are shown for the overall effect of toxin with placebo versus control (no toxin; pα) and the interaction between these effects and time (pβ). As described in materials and methods, for statistical analysis serial changes from baseline with lethal toxin were compared with serial changes from baseline in controls. However, for clarity in A–F, the serial effects of challenge (i.e., toxin minus control) are shown.
Comparison of the Effects of Lethal Toxin in Sprague-Dawley Versus Lewis Rat Hearts
The lethal toxin preparation used in this experiment (500 ng/ml) had limited effects on heart function in Sprague-Dawley rat hearts (data not shown). However, this preparation at a dose of 2,500 ng/ml decreased HR, dP/dtmax, and RPP significantly in both Sprague-Dawley and Lewis rat strains and significantly decreased LVDP and increased dP/dtmin in the latter (P ≤ 0.02; data not shown) but not the former. Changes in HR in Lewis rats were greater at later time points (P = 0.03 for the toxin effect and time interaction; data not shown).
DISCUSSION
Edema toxin had demonstrable effects in this perfused rat heart model at doses comparable to or less than those showing effects in vivo (6). In both the nonrecirculating and recirculating models, the lower edema toxin dose tested consistently increased HR, CF, LVDP, RPP, and dP/dtmax and decreased dP/dtmin (Figs. 2–5). These changes were likely associated with edema toxin's known stimulatory effects on intracellular cAMP (Fig. 6), since increased intracellular cAMP levels are known to produce chronotropic, inotropic, and vasodilatory effects (33). Consistent with this, PA mAb, which inhibits edema factor-associated cAMP stimulation in vitro (5, 21), reduced edema toxin's induced increases in myocardial and effluent cAMP (Fig. 6) and in HR and CF (Fig. 3). Also, adefovir, a nucleoside that competes with ATP for edema factor binding (27), had similar inhibitory effects (Fig. 4). Of note, while higher edema toxin doses produced early changes similar to those produced by the lower dose, these changes did not persist (Fig. 2) Thus, higher edema toxin doses, while likely also increasing cAMP levels early, may have subsequently reduced necessary substrate levels or elicited counterregulatory mechanisms that limited the continued expression of earlier stimulatory effects.
It is also noteworthy that while PA mAb and adefovir consistently and significantly reduced the effects of edema toxin on HR and CF, their influence on other myocardial parameters was less apparent. In the case of PA mAb, this may relate to an insufficient sample size, since treatment produced trends in reducing edema toxin-associated increases in RPP and dP/dtmax. The lack of significant effects of PA mAb or adefovir on myocardial contraction with edema toxin may also relate to the doses of the agents used. However, the ratio of PA mAb to protective antigen administered was highly protective against edema toxin and lethal toxin challenge in vivo (1, 7). In preliminary experiments with adefovir alone, higher doses manifested myocardial effects that would have confounded findings with edema toxin and were therefore not used. It is also possible that while the persistent effects of edema toxin on HR and CF are directly dependent on intracellular increases in cAMP, its inotropic effects require cAMP for activation but not continuation.
Dobutamine, an agent that also has cAMP-mediated myocardial effects (17, 33, 34), produced rapid changes in both inotropic and chronotropic heart functions but only during its administration (Fig. 5). In comparison, the myocardial effects of edema toxin were slower in development but then persisted after toxin administration was stopped. These differences may relate to the rate of cellular uptake and the stability of the agents. Dobutamine rapidly increases cAMP via β1-adrenergic receptor activation and transmembrane signaling, which then promptly stimulates contractility and HR changes (33). However, dobutamine is also rapidly metabolized, with a half-life of 2.4 min, and its stimulatory effects are transitory (17). In contrast, edema factor must first gain access to the cell cytoplasm via protective antigen-mediated membrane translocation and then be activated by calmodulin to catalyze the synthesis of cAMP (21). After exposure to edema toxin, maximal cAMP levels were not reached for up to 60 min in vivo and up to 90 min in our model (Fig. 6) (21). Furthermore, edema factor has a reported half-life of 2 h in the cytosol (19), which may explain edema toxin's persistent effects in the present experiments.
The increase in CF observed with edema toxin in the present study is of particular interest (Figs. 2–5). Treatment with recognized vasodilators like adenosine, bradykinin, and nitroprusside increase CF in constant-pressure models (10, 31), whereas vasoconstrictors like adrenaline or nitric oxide inhibitors decrease flow (3, 11). Thus, the increases in CF observed with edema toxin in the present study may reflect direct coronary vasodilation. This finding suggests that edema toxin could contribute to anthrax-associated shock by producing direct systemic arterial and venous dilation independent of its effects on extravasation of fluid. Consistent with this, edema toxin caused rapid reductions in central venous and arterial blood pressures and systemic vascular resistance in canines without hemoconcentration (30).
Lethal toxin administered at a dose comparable to one producing hypotension and mortality in vivo (6) had little observable effect on perfused heart function (Fig. 7). This finding is consistent with in vivo studies (8, 30) in which the hemodynamic changes of lethal toxin required 6 h or longer to develop. Whether the changes noted with the 10-fold greater lethal toxin dose used in the dose-response experiment are relevant to the toxin's in vivo effects is not clear. However, the results from the experiment comparing lethal toxin in Sprague-Dawley and Lewis hearts (experiment 7) do not support such a relationship. Sensitivity to lethal toxin-induced death in rats has been mapped to a single locus, rNlrp1(23). While variations in this genetic locus conferred differing survival benefits with lethal toxin in vivo, in experiment 7 rNlrp1 did not appear to differentially influence myocardial function. That is, hearts from the lethal toxin-resistant Lewis rat strain (expressing a Nlrp1R allele) had similar myocardial responses to high doses of toxin as those from toxin-sensitive Sprague-Dawley rats (expressing a Nlrp1S allele). Thus, protection with the Nlrp1R allele may involve nonmyocardial effects. Alternatively, the myocardial changes associated with these very high lethal toxin doses in the perfused heart model may not reflect those associated with lethality in vivo. A recent in vitro study (16) has suggested that lethal toxin may depress cardiomyocyte function via an NADPH oxidase-dependent mechanism.
Findings from this study have several clinical implications. They provide further evidence that edema toxin production during anthrax infection could contribute to hemodynamic dysfunction. Importantly, increases in CF raise the possibility that edema toxin produces direct systemic vasodilation. This toxin could also inhibit the effects of vasopressor agents that function via cAMP suppression (12, 32). It is less clear how the inotropic effects of edema toxin in the present study relate clinically. In canines, edema toxin doses producing hypotension and death did not alter LVEF (30). However, preload and afterload changes occurring in vivo may confound the interpretation of these LVEF measures. Finally, mechanisms underlying depressed myocardial function with the lethal toxin doses used in in vivo models appear to require more time to manifest themselves than could be observed in isolated perfused hearts. In conclusion, delineating the myocardial and peripheral vascular effects of both edema toxin and lethal toxin will be important for better understanding and managing anthrax-associated shock.
DISCLOSURES
M. Subramanian and T. S. Mignone are employed by Human Genome Science, Incorporated.
ACKNOWLEDGMENTS
The authors thank Dr. Anne Deschamps and Dr. Elizabeth Murphy (National Heart, Lung, and Blood Institute, National Institutes of Health) for helping assemble the rat heart Langendorff perfusion model.
REFERENCES
- 1.Altaweel L, Cui X, Su J, Solomon S, Li Y, Moayeri M, Chen Z, Fitz Y, Leppla S, Purcell R, Eichacker P. Chimpanzee derived monoclonal antibody against protective antigen improves survival in rats challenged with anthrax edema and lethal toxin (Abstract). Am J Respir Crit Care Med 177: A578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth MG, Hood J, Brooks TJ, Hart A. Anthrax infection in drug users. Lancet 375: 1345–1346, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bouma P, Ferdinandy P, Sipkema P, Allaart CP, Westerhof N. Nitric oxide is an important determinant of coronary flow in the isolated blood perfused rat heart. Basic Res Cardiol 87: 570–584, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Cheng CP, Masutani S, Cheng HJ, Cross M, Zhang CX, Zhou P, Cann J, Cline JM, Little WC, Kuo SR, Frankel AE. Progressive left ventricle, myocyte dysfunction, and heart failure in the lethality of anthrax toxin in conscious dogs. Circulation 116: 758–758, 2007 [Google Scholar]
- 5.Cooksey BA, Sampey GC, Pierre JL, Zhang X, Karwoski JD, Choi GH, Laird MW. Production of biologically active Bacillus anthracis edema factor in Escherichia coli. Biotechnol Prog 20: 1651–1659, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Cui X, Li Y, Li X, Laird MW, Subramanian M, Moayeri M, Leppla SH, Fitz Y, Su J, Sherer K, Eichacker PQ. Bacillus anthracis edema and lethal toxin have different hemodynamic effects but function together to worsen shock and outcome in a rat model. J Infect Dis 195: 572–580, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Cui X, Li Y, Moayeri M, Choi GH, Subramanian GM, Li X, Haley M, Fitz Y, Feng J, Banks SM, Leppla SH, Eichacker PQ. Late treatment with a protective antigen-directed monoclonal antibody improves hemodynamic function and survival in a lethal toxin-infused rat model of anthrax sepsis. J Infect Dis 191: 422–434, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cui X, Moayeri M, Li Y, Li X, Haley M, Fitz Y, Correa-Araujo R, Banks SM, Leppla SH, Eichacker PQ. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol 286: R699–R709, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Frankel AE, Kuo SR, Dostal D, Watson L, Duesbery NS, Cheng CP, Cheng HJ, Leppla SH. Pathophysiology of anthrax. Front Biosci 14: 4516–4524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita S, Roerig DL, Bosnjak ZJ, Stowe DF. Effects of vasodilators and perfusion pressure on coronary flow and simultaneous release of nitric oxide from guinea pig isolated hearts. Cardiovasc Res 38: 655–667, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Glomstein A, Hauge A, Oye I, Sinclair D. Effects of adrenaline on coronary flow in isolated perfused rat hearts. Acta Physiol Scand 69: 102–110, 1967 [DOI] [PubMed] [Google Scholar]
- 12.Gornemann T, Villalon CM, Centurion D, Pertz HH. Phenylephrine contracts porcine pulmonary veins via α1B-, α1D-, and α2-adrenoceptors. Eur J Pharmacol 613: 86–92, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Gwinn W, Zhang M, Mon S, Sampey D, Zukauskas D, Kassebaum C, Zmuda JF, Tsai A, Laird MW. Scalable purification of Bacillus anthracis protective antigen from Escherichia coli. Protein Expr Purif 45: 30–36, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hearse DJ, Sutherland FJ. Catecholamines and preconditioning: studies of contraction and function in isolated rat hearts. Am J Physiol Heart Circ Physiol 277: H136–H143, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 7: 933–944, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandadi MR, Hua Y, Ma H, Li Q, Kuo SR, Frankel AE, Ren J. Anthrax lethal toxin suppresses murine cardiomyocyte contractile function and intracellular Ca2+ handling via a NADPH oxidase-dependent mechanism. PLoS One 5: e13335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kates RE, Leier CV. Dobutamine pharmacokinetics in severe heart failure. Clin Pharmacol Ther 24: 537–541, 1978 [DOI] [PubMed] [Google Scholar]
- 18.Kochi SK, Schiavo G, Mock M, Montecucco C. Zinc content of the Bacillus anthracis lethal factor. FEMS Microbiol Lett 124: 343–348, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Krakauer T, Little SF, Stiles BG. Bacillus anthracis edema toxin inhibits Staphylococcus aureus enterotoxin B effects in vitro: a potential protein therapeutic? Infect Immun 73: 7069–7073, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laird MW, Zukauskas D, Johnson K, Sampey GC, Olsen H, Garcia A, Karwoski JD, Cooksey BA, Choi GH, Askins J, Tsai A, Pierre J, Gwinn W. Production and purification of Bacillus anthracis protective antigen from Escherichia coli. Protein Expr Purif 38: 145–152, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA 79: 3162–3166, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moayeri M, Crown D, Dorward DW, Gardner D, Ward JM, Li Y, Cui X, Eichacker P, Leppla SH. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS). PLoS Pathog 5: e1000456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman ZL, Printz MP, Liu S, Crown D, Breen L, Miller-Randolph S, Flodman P, Leppla SH, Moayeri M. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog 6: e1000906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Leppla SH. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr Purif 18: 293–302, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Puhar A, Dal Molin F, Horvath S, Ladant D, Montecucco C. Anthrax edema toxin modulates PKA- and CREB-dependent signaling in two phases. PLoS One 3: e3564, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez DM, Leppla SH, Schneerson R, Shiloach J. Production, recovery and immunogenicity of the protective antigen from a recombinant strain of Bacillus anthracis. J Ind Microbiol Biotechnol 28: 232–238, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Shen Y, Zhukovskaya NL, Zimmer MI, Soelaiman S, Bergson P, Wang CR, Gibbs CS, Tang WJ. Selective inhibition of anthrax edema factor by adefovir, a drug for chronic hepatitis B virus infection. Proc Natl Acad Sci USA 101: 3242–3247, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherer K, Li Y, Cui X, Eichacker PQ. Lethal and edema toxins in the pathogenesis of Bacillus anthracis septic shock: implications for therapy. Am J Respir Crit Care Med 175: 211–221, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res 41: 613–627, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Sweeney D, Cui X, Solomon S, Vitberg D, Migone T, Scher D, Danner R, Natanson C, Subramanian M, Eichacker P. Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. J Infect Dis 202: 1885–1896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talukder MA, Morrison RR, Mustafa SJ. Comparison of the vascular effects of adenosine in isolated mouse heart and aorta. Am J Physiol Heart Circ Physiol 282: H49–H57, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Taylor MS, Benoit JN. Effect of milrinone on small mesenteric artery vasoconstriction: role of K+ channels. Am J Physiol Gastrointest Liver Physiol 277: G69–G78, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Topol EJ, Califf RM. Textbook of Cardiovascular Medicine. Philadelphia, PA: Lippincott, Williams & Wilkins, 2007 [Google Scholar]
- 34.Tuttle RR, Mills J. Dobutamine: development of a new catecholamine to selectively increase cardiac contractility. Circ Res 36: 185–196, 1975 [DOI] [PubMed] [Google Scholar]
- 35.Wang QD, Tokuno S, Valen G, Sjoquist PO, Thoren P. Cyclic fluctuations in the cardiac performance of the isolated Langendorff-perfused mouse heart: pyruvate abolishes the fluctuations and has an anti-ischaemic effect. Acta Physiol Scand 175: 279–287, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Watson LE, Kuo SR, Katki K, Dang T, Park SK, Dostal DE, Tang WJ, Leppla SH, Frankel AE. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS One 2: e466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson LE, Mock J, Lal H, Lu G, Bourdeau RW, Tang WJ, Leppla SH, Dostal DE, Frankel AE. Lethal and edema toxins of anthrax induce distinct hemodynamic dysfunction. Front Biosci 12: 4670–4675, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem 76: 243–265, 2007 [DOI] [PubMed] [Google Scholar]