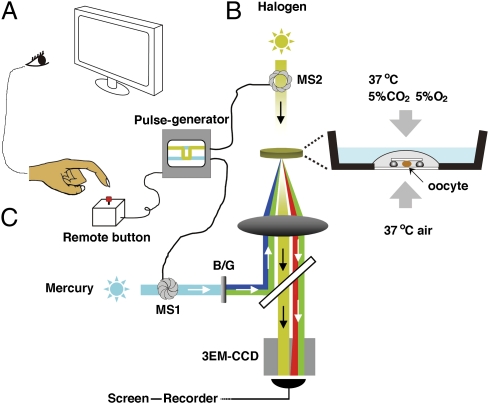
Fig. 1.
The video microscopic in vitro fertilization system consists of three components. (A) Oocyte-holding Petri dish (Materials and Methods). An oocyte surrounded by cumulus cell mass was slightly compressed under a 3 × 3 mm coverslip supported by four silicon grease spots. The dish was placed into an incubation chamber supplied with a mixed gas (5% CO2, 5% O2, and 90% N2). (B) Mercury–halogen illumination system: two sets of mechanical shutters (VS25; Uniblitz), one near the mercury lamp (MS1) and the other next to the halogen lamp (MS2), were controlled via a pulse generator. (C) Image-capturing system: a supersensitive video camera (NC-R550b; NEC) and a blue/green dual bandpass filter set enabled simultaneous visualization of EGFP and Ds-Red2 fluorescence in swimming spermatozoa. All spermatozoa entering the cumulus were viewed first at low magnification (10× objective lens). When a spermatozoon was seen approaching the oocyte, it was then focused under higher magnification (20× objective lens; Fig. S6). The spermatozoon under observation was exposed to blue/green light intermittently (125 ms) by pressing a remote shutter control button. After about 90 min of recording, all of the spermatozoa were individually analyzed for the status of their acrosomes. Motile (live) spermatozoa with EGFP fluorescence in their acrosomes were considered “acrosome intact” and those without EGFP fluorescence were considered “acrosome reacted.”