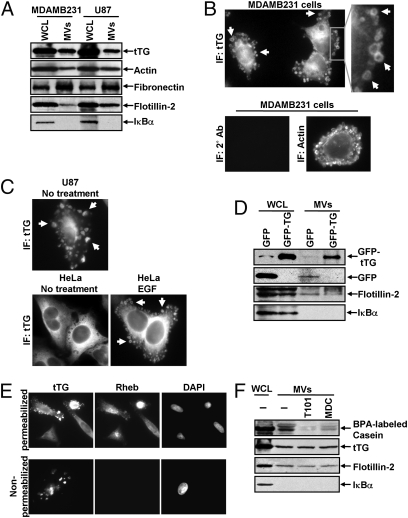
Fig. 3.
MV shed by cancer cells contain tTG. (A) WCL of serum-starved MDAMB231 and U87 cells, as well as lysates of the MV shed by these cells, were immunoblotted with several antibodies, including one against tTG. (B) (Upper Left) MDAMB231 cells immunostained with a tTG antibody. (Upper Right) The boxed area was enlarged, and arrows indicated certain MV. (Lower) An MDAMB231 cell costained with only the secondary antibody (Left) and with rhodamine-conjugated phalloidin to label the MV (Right). (C) Images of serum-starved U87 glioma cells and HeLa cervical carcinoma cells that were left untreated or were stimulated with EGF for 15 min as indicated and then were immunostained with a tTG antibody. Pronounced MV are indicated by arrows. (D) WCL of MDAMB231 cells ectopically expressing either GFP only or GFP-tTG, as well as lysates of the MV shed by these transfectants into their culturing medium, were immunoblotted with antibodies against GFP, the MV marker flotillin-2, and the cytosolic-specific marker IκBα. (E) Fluorescent images of permeabilized and nonpermeabilized samples of MDAMB231 cells stained with antibodies against tTG, the intracellular protein Ras homolog enriched in brain (Rheb), and DAPI to label nuclei. (F) WCL of serum-starved MDAMB231 cells and intact MV generated by these cells treated with or without the tTG inhibitors T101 (cell impermeable) or MDC (cell permeable), were assayed for transamidation activity as read out by the incorporation of BPA into casein. The samples then were immunoblotted with antibodies against tTG, flotillin-2, and IκBα.