Abstract
Purpose
This study examines the role of cell survival/apoptosis related proteins involved in NFκB signaling pathways and its associated events in GTP-induced chemoprevention of prostate cancer in TRAMP mice.
Methods
Mice were given 0.1% GTP as drinking fluid. Western blot and immunohistochemical analysis performed to examine NFκB and its regulated pathway in response to GTP.
Results
Our data demonstrated increased expression of NFκB, IKKα, IKKβ, RANK, NIK and STAT-3 in dorso-lateral prostate of TRAMP mice as a function of age and tumor growth and continuous GTP infusion for 32 weeks resulted in substantial reduction in these proteins. The levels of transcription factor osteopontin, a non-collagenous extracellular matrix protein, were also downregulated. Inhibition of NFκB signaling is known to activate apoptotic and inhibit anti-apoptotic proteins. Therefore, we analyzed Bax and Bcl2 levels in the dorsolateral prostate of TRAMP mice fed GTP and observed a shift in balance between Bax and Bcl2 favoring apoptosis.
Conclusions
Based on the data we suggest that oral consumption of GTP might inhibit osteopontin and NFκB signaling that may contribute to induction of apoptosis observed in GTP fed TRAMP mice.
Keywords: green tea, NFκB, osteopontin, RANK, TRAMP
INTRODUCTION
Cancer of the prostate gland (PCa) is one of the most common non-cutaneous cancers of men and is currently the second leading cause of cancer related deaths in the United States (1,2) with similar trends in other western countries (3–5). In the absence of satisfactory treatment options for PCa, chemoprevention could be an effective approach to reduce the incidence of the disease, an area of investigation which we recently reviewed (6 and the references therein). PCa represents an ideal candidate disease for chemoprevention because it is diagnosed in elderly males; thus even a moderate delay in the neoplastic changes achieved through pharmacologic or dietary intervention could result in a substantial reduction in the incidence of the clinically detectable disease (6–8). There is currently intense effort in identifying mechanism-based naturally occurring agents present in diet and beverages consumed by humans for their cancer chemopreventive and possibly cancer therapeutic efficacy against PCa (6–9). The identification of new predictive biomarkers will be important for improving clinical management, outcome of treatment protocols, and assessing the effectiveness of chemopreventive regimens, all of which could lead to improved survival of patients with the disease. Molecular targets for PCa, especially those that are indicative of invasiveness of the disease, will be excellent candidate targets for staging the disease and establishing effectiveness of chemoprevention of PCa. Among these agents, reports from this laboratory and elsewhere (6,7,10–12) have shown that polyphenols derived from green tea (GTP) are potential chemopreventive agents against PCa. We have earlier shown that oral infusion of GTP to transgenic adenocarcinoma of the mouse prostate (TRAMP), inhibits the development of PCa and identified that IGF-I/IGFBP-3 signaling pathway as a prime pathway for GTP-mediated inhibition of PCa that limits the progression of cancer through inhibition of angiogenesis and metastasis (12).
IGF-1 is recognized to stimulate a continuous activation of NFκB signaling which in turn modulates its downstream and other associated cellular events (13,14). NFκB plays an important role in cancer as it has been documented to be involved in variety of cellular events. Understanding the molecular mechanisms of GTP-mediated inhibition of PCa is essential in devising rationale and mechanism-based chemopreventive approaches. Here, we show that green tea polyphenol-induced suppression of PCa progression may be mediated through inhibition of NFκB and its linked events.
MATERIALS AND METHODS
Reagents
Antibody against RANK, NIK, IKK-α, IKKβ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), antibody against Stat-3 from Cell Signaling Technology (Danvers, MA, USA), antibody against Osteopontin from Abcam (Cambridge, MA, USA) and anti β-actin was obtained from Sigma (St. Louis, MO, USA). The bicinchoninic acid (BCA) protein assay kit was obtained from Pierce Biotechnology (Rockford, IL, USA) Novex precast Tris glycine gels were obtained from Invitrogen (Carlsbad, CA, USA).
Animals
The male and female heterozygous C57BL/TGN TRAMP mice, Line PB Tag 8247NG, were bred and maintained in the Animal Care Facility of University of Wisconsin, School of Medicine. Housing and care of the animals was in accordance with the guidelines established by the University’s Animal Research Committee consistent with the NIH Guidelines for the Care and Use of Laboratory Animals. Transgenic males and non-transgenic littermates for these studies were routinely obtained as [TRAMP × C57BL/6] F1 or as [TRAMP × C57BL/6] F2 offspring. Isolation of mouse-tail DNA and PCR-based screening assay were performed as described previously (15).
Study Design and Green Tea Polyphenol Supplementation
Green tea polyphenols (GTP >95% enriched preparation) were obtained from Natural Resources & Products (Charlottesville, VA, USA). Throughout the experiment, the animals were housed under standard animal housing conditions and had free access to laboratory chow ad libitum. As described previously (12), freshly prepared solution of 0.1% GTP in tap water was supplied every Monday, Wednesday, and Friday to experimental animals as the sole source of drinking fluid for 24 weeks (green tea polyphenol-fed group), and the control (water-fed group) animals were supplied with the same tap water throughout the experiment. This feeding regimen is well tolerated by animals and has been used in mice in many previous studies from this and other laboratories and is equivalent to an approximate consumption of six cups of green tea per day by an average adult human (16). Forty 4-week-old male TRAMP mice were divided into two equal groups consisting of 20 animals in water-fed control group and 20 in GTP-fed treated group. At each time point i.e. 8, 16, 24 and 32 weeks, five mice were removed and killed by cervical dislocation, and the dorso-lateral prostate was removed under a dissecting microscope for biochemical and histological analysis. Water fed non-transgenic littermates were run in parallel as controls.
Immunoblot Analysis
The dorso-lateral prostate removed from both treated and control groups was homogenized in lysis buffer [50 mmol/l Tris–HCl, 150 mmol/l NaCl, 1 mmol/l EGTA, 1 mmol/l EDTA, 20 mmol/l NaF, 100 mmol/l Na3VO4, 0.5% NP40, 1% Triton X-100, 1 mmol/l phenylmethylsulfonyl fluoride, 10 mg/ml aprotinin, and 10 mg/ml leupeptin (pH 7.4)] at 4°C to prepare cell lysates. The protein concentration was determined by BCA assay using the manufacturer’s protocol and western blot was performed as previously described (17).
Densitometric Analysis
Immunoblots were scanned by HP Precisionscan Pro 3.13 (Hewlett-Packard Co., Palo Alto, CA, USA). Densitometry measurements of the scanned bands were performed using digitalized scientific software program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT, USA). Data were normalized to β-actin or suitable loading controls and expressed as mean±SEM followed by appropriate statistical analysis.
Statistical Analysis
Results were analyzed using a two-tailed Student’s t test to assess statistical significance. To assess change in protein expression during the course of cancer progression, comparisons were made with water-fed animals of the preceding age. To access the effect of oral feeding of GTP, comparisons were made with age-matched water-fed TRAMP mice. Values of P<0.05 were considered statistically significant.
RESULTS
Effect of Green Tea Infusion on Nuclear Factor Kappa B Pathway During Progressive Stages of Prostate Cancer Development in TRAMP Mice
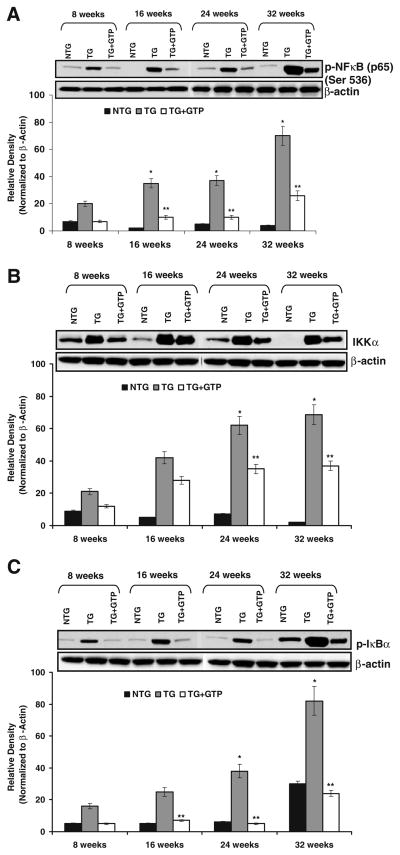
Because NFκB is considered to play an important role in cancer chemoprevention due to its involvement in a number of cellular events like tumor cell growth, proliferation, angiogenesis, invasion, apoptosis, and survival. We examined the effect of oral administration of GTP on the constitutive activation of NFκB in dorso-lateral prostate tissue of TRAMP mice of increasing age and cancer progression (Fig. 1A). We observed a progressive increase in the phosphorylation of NFκB at Ser536 as cancer progressed from not detectable cancer at 8 weeks to well differentiated adenocarcinoma at 16 weeks to moderately differentiated adenocarcinoma at 24 weeks and finally to poorly differentiated adenocarcinoma at 32 weeks of age. The phosphorylation of NFκB in the non-transgenic littermates was not significant. As shown in Fig. 1A, continuous GTP infusion to TRAMP mice resulted in significant inhibition in the protein expression of p-NFκB at all time points of prostate cancer progression as compared to water infused TRAMP mice. NFκB is a ubiquitous nuclear transcription factor that plays a major regulatory role in carcinogenesis. In the absence of stimulatory signals it resides in the inactive state in the cytoplasm as a heterotrimer consisting of p50, p65, and IκBα subunits. IKK has been identified that phosphorylates serine residues in IκBα. The phosphorylation and proteolytic degradation of IκBα results in activation and nuclear translocation of NFκB. We observed that IKKα protein expressions were significantly lowered by infusion of GTP at all the time points (Fig. 1B), we also observed a highly significant inhibition in the phosphorylation of IκBα in dorso-lateral prostate of TRAMP mice that were given green tea as sole source of drinking fluid (Fig. 1C).
Fig. 1.
Effect of GTP infusion on p-NFκB, IKKα, and p-IκBα in the dorso-lateral prostate of TRAMP mice during progressive stages of PCa development. Protein levels by immunoblot analysis of A p-NFκB (p65) at Ser536, B IKKα and C p-IκBα. As detailed in “MATERIALS AND METHODS”, the protein levels were determined in the dorso-lateral prostate of 8-, 16-, 24-, and 32-week-old control (water-fed) and treated (GTP-fed) TRAMP mice along with their age matched non-transgenic littermates. Equal loading of protein was confirmed by stripping the blot and re-probing with β-actin antibody. Western blot analysis was conducted in five animals in each group, and only representative blots are shown. Histogram represents relative density data of the immunoblots in relative units ± SEM normalized to β-actin. *P<0.05; compared with water-fed TRAMP mice of the preceding age. **P<0.001 compared with age matched water-fed TRAMP mice.
Effect of Green Tea Infusion on Receptor Activator of Nuclear Factor Kappa B and Nuclear Factor Kappa B Inducing Kinase During Progressive Stages of Prostate Cancer Development in TRAMP Mice
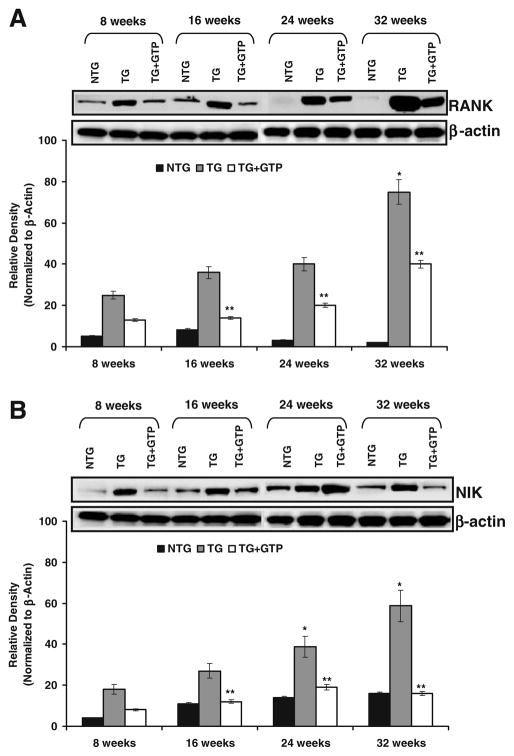
The receptor activator of NFκB (RANK) is a member of TNF-receptor super family that is known to induce osteoclastogenesis and dendritic cell survival and plays a role in growth inhibition and apoptosis (18). This receptor can interact with various TRAF family proteins, through which this receptor induces the activation of NFκB and MAPK8/JNK. As shown in Fig. 2A, we observed a decreased protein expression of RANK in the dorsolateral prostate of green tea polyphenol-fed TRAMP mice compared with the age dependent increase of the protein in water-fed control group. RANK-mediated NF-κB activation proceeds via a novel TRAF6 interaction motif, which then activates NF-κB Inducing Kinase (NIK), subsequently leading to NF-κB activation. We observed age dependent increase in the protein expression of NIK which was subsequently inhibited by oral infusion of green tea to the TRAMP mice (Fig. 2B). The protein expression of RANK and NIK were not observed to be significant in the non-transgenic littermates (Fig. 2A,B).
Fig. 2.
Effect of GTP infusion on RANK and NIK in the dorso-lateral prostate of TRAMP mice during progressive stages of PCa development. Protein levels by immunoblot analysis of A RANK and B NIK. As detailed in “MATERIALS AND METHODS”, the protein levels were determined in the dorso-lateral prostate of 8-, 16-, 24-, and 32-week-old control (water-fed) and treated (GTP-fed) TRAMP mice along with their age matched non-transgenic littermates. Equal loading of protein was confirmed by stripping the blot and re-probing with β-actin antibody. Western blot analysis was conducted in five animals in each group, and only representative blots are shown. Histogram represents relative density data of the immunoblots in relative units ± SEM normalized to β-actin. *P<0.05; compared with water-fed TRAMP mice of the preceding age. **P<0.001 compared with age matched water-fed TRAMP mice.
Effect of Green Tea Infusion on Osteopontin and STAT3 During Progressive Stages of Prostate Cancer Development in TRAMP Mice
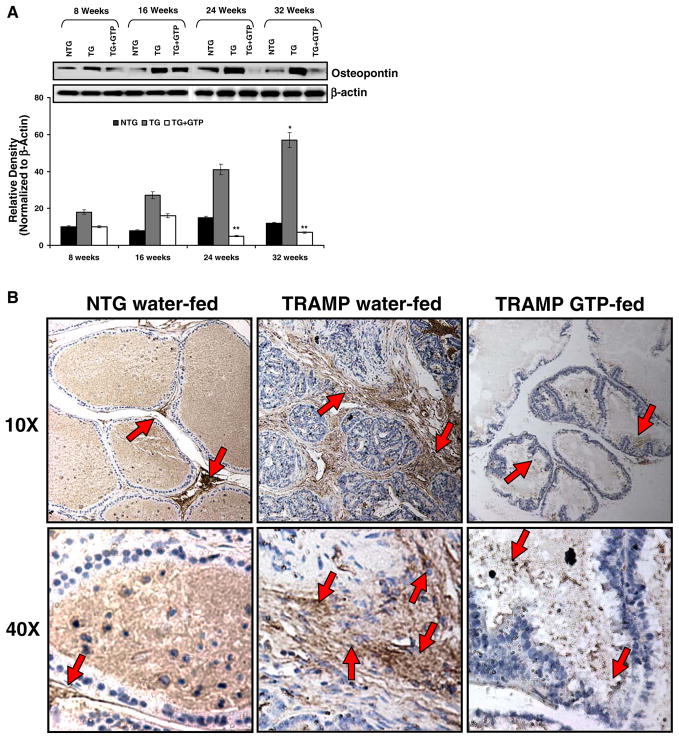
Osteopontin is a secreted glycosylated phosphoprotein known to be associated with the progression of several cancers including cancer of the prostate gland (19). Over-expression of osteopontin contributes to a proliferative advantage in PCa cells and also influences their in-vitro invasive ability. We observed a comprehensive up-regulation in the protein expression of this protein as the cancer progressed in TRAMP mice (Fig. 3A). Continuous GTP infusion to these mice resulted in significant inhibition in the protein expression of osteopontin at all the time points tested. Similar results were observed by immunohistochemical analysis of the prostate tissue of TRAMP mice (Fig. 3B). Mice with poorly differentiated adenocarcinoma (32 weeks) exhibited strong staining for osteopontin. This staining was especially seen in the stroma and also in the epithelia of prostatic acini. Continuous GTP infusion to these mice resulted in significant inhibition in the expression of osteopontin.
Fig. 3.
Effect of GTP infusion on osteopontin protein levels in the dorso-lateral prostate during progressive stages of PCa development in TRAMP mice. A protein levels of osteopontin by immunoblot analyses. As detailed in “MATERIALS AND METHODS”, the protein levels were determined in the dorso-lateral prostate of water-fed control and green tea polyphenol-fed TRAMP mice at 8, 16, 24, and 32 weeks of age with their non-transgenic littermates. Western blot analysis was conducted in five animals in each group, and only representative blots are shown. Histogram represents relative density data of the immunoblots in relative units ± SEM normalized to β-actin. *P<0.05 compared with water-fed TRAMP mice of the preceding age. **P<0.001 compared with age matched water-fed TRAMP mice. B protein levels of osteopontin by immunohistochemical analyses. As detailed in “MATERIALS AND METHODS”, the protein levels were determined in the dorso-lateral prostate of non-transgenic, water-fed control and green tea polyphenol-fed TRAMP mice at 24 weeks of age. Representative photomicrographs (magnification, ×10 and ×40) of immunohistochemical staining for osteopontin in non-transgenic, water-fed control and GTP-fed TRAMP mice. Immunostaining data were confirmed in two slides from five animals.
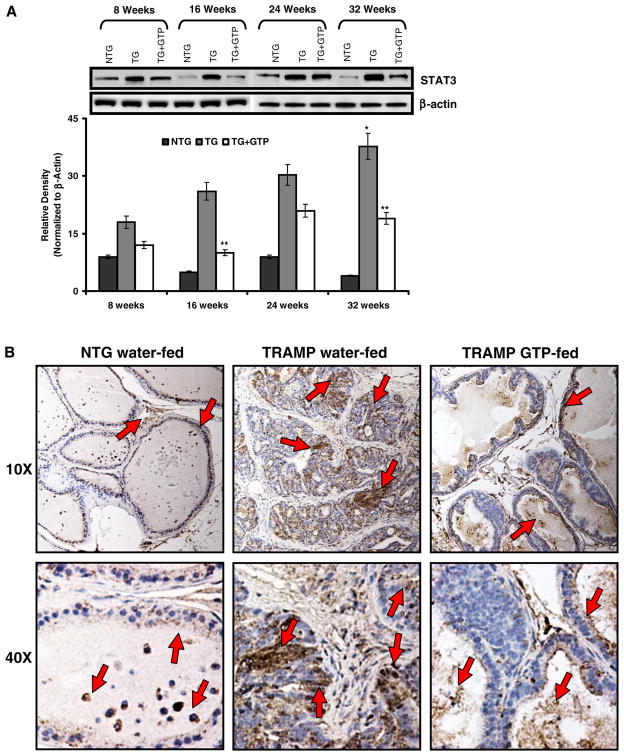
Persistent activation of signal transducer and activator of transcription (STAT-3) is a common feature of PCa (20,21) and increased resistance to apoptosis occurs due to constitutive activation of STAT-3. Western blot analysis was performed to look at the protein expression of STAT-3, we observed a consistent upregulation of the protein as the tumor progressed from not-detectable at 8 weeks to poorly-differentiated at 32 weeks in dorsolateral prostate of TRAMP mice. This upregulation was significantly inhibited in age matched mice with continuous GTP infusion (Fig. 4A). These results were further confirmed by immunohistochemical analysis of STAT-3 levels (Fig. 4B), indicating a significant decrease in STAT-3 protein expression in green tea polyphenol-fed TRAMP mice.
Fig. 4.
Effect of GTP infusion STAT3 protein levels in the dorso-lateral prostate during progressive stages of PCa development in TRAMP mice. A protein levels of STAT3 by immunoblot analyses. As detailed in “MATERIALS AND METHODS”, the protein levels were determined in the dorso-lateral prostate of water-fed control and green tea polyphenol-fed TRAMP mice at 8, 16, 24, and 32 weeks of age with their non-transgenic littermates. Western blot analysis was conducted in five animals in each group, and only representative blots are shown. Histogram represents relative density data of the immunoblots in relative units ± SEM normalized to β-actin. *P<0.05 compared with water-fed TRAMP mice of the preceding age. **P<0.001 compared with age matched water-fed TRAMP mice. B protein levels of STAT3 by immunohistochemical analyses. As detailed in “MATERIALS AND METHODS”, the protein levels were determined in the dorso-lateral prostate of non-transgenic, water-fed control and GTP-fed TRAMP mice at 24 weeks of age. Representative photomicrographs (magnification, ×10 and ×40) of immunohistochemical staining for STAT3 in non-transgenic, water-fed control and GTP-fed TRAMP mice. Immunostaining data were confirmed in two slides from five animals.
Effect of Green Tea Infusion on bax/bcl2 Ratio During Progressive Stages of Prostate Cancer Development in TRAMP Mice
We earlier demonstrated that oral infusion of GTP to TRAMP mice is associated with an induction of apoptosis (16). The ratio of the proapoptotic Bax and anti-apoptotic Bcl2 is critically balanced during cell proliferation such that an increase in the levels of Bax or a decrease in the level of Bcl2 can shift the ratio and trigger a signal initiating apoptosis. The expression of the anti-apoptotic protein Bcl2 is positively regulated by the transcription factor NFκB. Since GTP infusion resulted in modulation of NFκB and its regulated pathway, we hypothesized that EGCG treatment would result in altered levels of the Bcl2 family of proteins. To test our hypothesis, we determined the protein levels of Bax and Bcl2 in dorsolateral prostate of TRAMP mice given oral infusion of GTP. We observed that GTP infusion induced a decrease in Bcl2 levels with a concomitant increase in Bax levels (Fig. 5). Densitometry of the immunoblot indicated that the ratio of Bax/Bcl2 was higher in GTP infused mice prostate compared to the water fed mice, thus supporting our contention that a shift in the Bax/Bcl-2 ratio activated the apoptotic pathway.
Fig. 5.
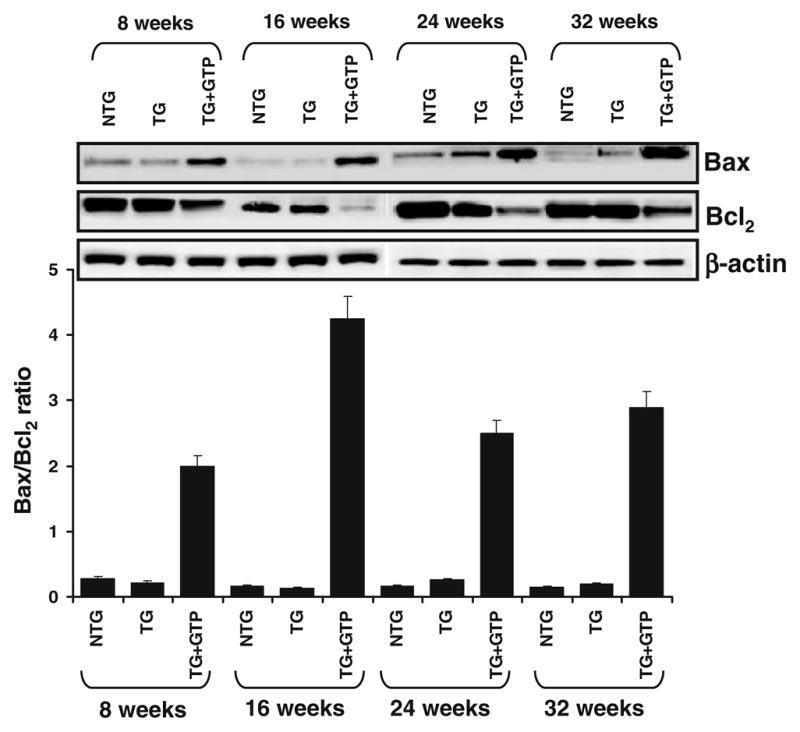
Effect of GTP infusion on Bax and Bcl2 protein levels in the dorso-lateral prostate of TRAMP mice during progressive stages of PCa development. Protein levels of Bax and Bcl2 by immunoblot analysis. As detailed in “MATERIALS AND METHODS”, the protein levels were determined in the dorso-lateral prostate of 8-, 16-, 24-, and 32-week-old control (water-fed) and treated (GTP-fed) TRAMP mice along with their age matched non-transgenic littermates. Equal loading of protein was confirmed by stripping the blot and re-probing with β-actin antibody. Western blot analysis was conducted in five animals in each group, and only representative blots are shown. Histogram indicates the ratio between Bax and Bcl2 obtained by densitometric analysis of the bands shown above normalized to β-actin.
DISCUSSION
The limited available options for the treatment of PCa have prompted the need for developing alternative strategies for the management of the disease. Chemoprevention by the use of dietary or nontoxic synthetic agents has offered a viable option to block neoplastic inception or delay disease progression. We earlier demonstrated that oral infusion of GTP at a human achievable dose (equivalent to six cups of green tea per day) inhibits the development of PCa and its metastasis in TRAMP mice (16) and identified that IGF-I/IGFBP-3 signaling pathway is a prime pathway for GTP-mediated inhibition of PCa and its metastasis in TRAMP mice (12).
It is well recognized that IGF-I stimulates a sustained activation of NFκB as the tumor progresses and constitutive activation of NFκB is observed in a time dependent manner in human and TRAMP. In the present study we investigated the role of GTP in suppressing prostate carcinogenesis in TRAMP mice. As development of therapy resistant PCa is often associated with constitutive activation of NFκB, we investigated the ability of GTP (which is known to induce apoptosis in a variety of human cancer cells) to inhibit NFκB activation in TRAMP mice model, which closely mimics the progression of the human disease. To initiate, we evaluated NFκB protein expression in the dorsolateral prostate of age matched TRAMP mice that were given water (control group) or GTP (treated group) as sole source of drinking fluid. We conducted experiments at time periods that represent various stages of tumor development. At 8 weeks there is no detectable cancer which progressively develops into well differentiated adenocarcinoma at 16 weeks to moderately differentiated adenocarcinoma at 24 weeks and finally to poorly differentiated adenocarcinoma at 32 weeks of age. We observed that as cancer progressed from undetectable cancer at 8 weeks to poorly differentiated adenocarcinoma at 32 weeks, there was a considerable upregulation in the phosphorylation of NFκB at Ser536. These results are consistent with previous observation where NFκB was observed to be upregulated, with increasing age in TRAMP mice (22) and prostate carcinoma cells (23,24). This up-regulation of this transcription factor was significantly inhibited (P<0.001) in the groups given GTP in drinking water (Fig. 1A). To further test the effect of GTP on the activation of NFκB, we evaluated the levels of IKK which frees NFκB by inactivating its repressor and consequently allows it to shuttle to nucleus where it up regulates the expression of target genes. Interestingly in non transgenic littermates we observed a decrease in IKK protein levels from 8th to 24 weeks of age indicating a progressive decrease in the cellular requirement of NFκB activation in-vivo. However, significant upregulation of IKK levels was observed as a function of age in TRAMP mice and GTP treatment led to further inhibition of IKK levels indicating an opposing role of GTP in NFκB activation similar to the observed inhibition of IKK isoforms by GTP, EGCG in osteosarcoma cells (25). As the IKK phosphorylates and deactivates IκB, the inhibitory component of NFκB signaling and hence plays important role in NFκB activation, with the increase in IKK expression level in TRAMP, a concomitant increase in IκB phosphorylation was found however GTP infusion strongly repressed this phosphorylation at 8th, 16th and 24th and 32nd weeks of age. These results point towards a crucial role of GTP in repressing NFκB activation primarily via 1) repressing its release from the inhibitor IκB and 2) repressing the ser536 activating phosphorylation in its activation loop and hence repressing its transactivation function. To further test the effect of GTP on TRAMP, we focused on other key molecules which regulate the activation of NFκB signaling pathway. Receptor activator of NFκB (RANK) is a recently cloned member of the TNFR superfamily with no significant homology to other members of this family. RANK ligand binds to RANK on dendritic cells, up regulates the expression of anti-apoptotic protein BcL-XL suggesting a role in dendritic cell survival. Its cytoplasmic domain interacts with TRAF2, TRAF5 and TRAF6 which in turn activate NFκB inducing kinase (NIK). An interesting finding here was the decrease in RANK expression in dorso lateral prostate of non transgenic littermates in an age dependent manner. TRAMP mice, in contrast exhibited an increase in RANK expression as a function of age indicating its role in bypassing cell growth inhibition and cell cycle arrest, treatment however of GTP to TRAMP mice led to a repression of RANK expression indicating an indirect link to inhibit NFκB function. We also found consequent increase in Bcl2 expression suggesting that RANK also up regulates Bcl2 expression in TRAMP mice similar to that observed in dendritic cells. Orally infused GTP efficiently inhibited Bcl2 expression in dorso-lateral prostate of TRAMP at different ages. Intriguingly, we found a dose dependent increase in NIK expression not only in TRAMP mice but also in their non transgenic littermates indicating a non redundant role of NIK in cell survival. Here although the increase was dose dependent, the expression of NIK was more robust in TRAMP prostate. As osteopontin plays important role in inflammation and some splice variants are specific for cancers, we tested the effect of GTP oral infusion on the TRAMP mice. Although, its steady state level is maintained throughout different ages (8, 16, 24 and 32 weeks) in non transgenic, a robust age dependent increase was observed in osteopontin expression in TRAMP mice. Here, oral infusion of GTP showed a very potent inhibition of osteopontin expression in TRAMP mice dorso lateral prostate tissue. In order to further explain the inhibitory effect of GTP on prostate carcinogenesis, we evaluated the expression of STAT3, whose constitutive activation in cells leads to the resistance of apoptosis (26,27). As expected, a concordant increase in STAT3 expression was found in TRAMP in contrast to steady state levels maintained in non transgenic littermates of various age. GTP infusion however had a profound effect on STAT expression at all time points tested. Taken together this study highlights the chemo-preventive effects of oral infusion of GTP in TRAMP mice. We suggest that the observed inhibition of NFκB signaling pathway along with STAT3 inhibition may potentially be involved in repressing tumor progression in TRAMP mice.
Acknowledgments
This original work for author’s laboratory was supported by US PHS Grants RO1 CA 78809; RO1 CA 101039, RO1 CA 120451 and O’Brian center Grant P50 DK065303-01.
References
- 1.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, Henley J, Liff J, Thun MJ. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int. 2002;90:162–173. doi: 10.1046/j.1464-410x.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- 5.Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. 2005;41:834–845. doi: 10.1016/j.ejca.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui IA, Saleem M, Adhami VM, Asim M, Mukhtar H. Tea beverage in chemoprevention and chemotherapy of prostate cancer. Acta Pharmacol Sin. 2007;28:1392–1408. doi: 10.1111/j.1745-7254.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 8.Canby-Hagino ED, Thompson IM. Mechanisms of disease: prostate cancer—a model for cancer chemoprevention in clinical practice. Nat Clin Pract Oncol. 2005;2:255–261. doi: 10.1038/ncponc0172. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui IA, Afaq F, Adhami VM, Mukhtar H. Prevention of prostate cancer through custom tailoring of chemopreventive regimen. Chem Biol Interact. 2008;171:122–132. doi: 10.1016/j.cbi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 11.Neill MG, Fleshner NE. An update on chemoprevention strategies in prostate cancer for 2006. Curr Opin Urol. 2006;16:132–137. doi: 10.1097/01.mou.0000193388.31727.d2. [DOI] [PubMed] [Google Scholar]
- 12.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Liu Y, Lowe WL., Jr The role of phosphatidylinositol 3-kinase and the mitogen-activated protein kinases in insulin-like growth factor-I-mediated effects in vascular endothelial cells. Endocrinology. 2001;142:1710–1719. doi: 10.1210/endo.142.5.8136. [DOI] [PubMed] [Google Scholar]
- 14.Garrouste F, Remacle-Bonnet M, Fauriat C, Marvaldi J, Luis J, Pommier G. Prevention of cytokine-induced apoptosis by insulin-like growth factor-I is independent of cell adhesion molecules in HT29-D4 colon carcinoma cells-evidence for a NF-kappaB-dependent survival mechanism. Cell Death Differ. 2002;9:768–779. doi: 10.1038/sj.cdd.4401022. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui IA, Zaman N, Aziz MH, Reagan-Shaw SR, Sarfaraz S, Adhami VM, Ahmad N, Raisuddin S, Mukhtar H. Inhibition of CWR22Rnu1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006;27:833–839. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]
- 18.Bharti AC, Takada Y, Shishodia S, Aggarwal BB. Evidence that receptor activator of nuclear factor (NF)-kappaB ligand can suppress cell proliferation and induce apoptosis through activation of a NF-kappaB-independent and TRAF6-dependent mechanism. J Biol Chem. 2004;279:6065–6076. doi: 10.1074/jbc.M308062200. [DOI] [PubMed] [Google Scholar]
- 19.Forootan SS, Foster CS, Aachi VR, Adamson J, Smith PH, Lin K, Ke Y. Prognostic significance of osteopontin expression in human prostate cancer. Int J Cancer. 2006;118:2255–2261. doi: 10.1002/ijc.21619. [DOI] [PubMed] [Google Scholar]
- 20.Barton BE, Karras JG, Murphy TF, Barton A, Huang HF. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol Cancer Ther. 2004;3:11–20. [PubMed] [Google Scholar]
- 21.Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK. Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- 22.Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64:224–239. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
- 23.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gélinas C, Rabson AB. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;52:183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- 24.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 25.Hafeez BB, Ahmed S, Wang N, Gupta S, Zhang A, Haqqi TM. Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with downregulation of nuclear factor-kappaB. Toxicol Appl Pharmacol. 2006;216:11–19. doi: 10.1016/j.taap.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Bromberg J. Signal transducers and activators of transcription as regulators of growth, apoptosis and breast development. Breast Cancer Res. 2000;2:86–90. doi: 10.1186/bcr38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedranzini L, Leitch A, Bromberg J. Stat3 is required for the development of skin cancer. J Clin Invest. 2004;114:619–622. doi: 10.1172/JCI22800. [DOI] [PMC free article] [PubMed] [Google Scholar]