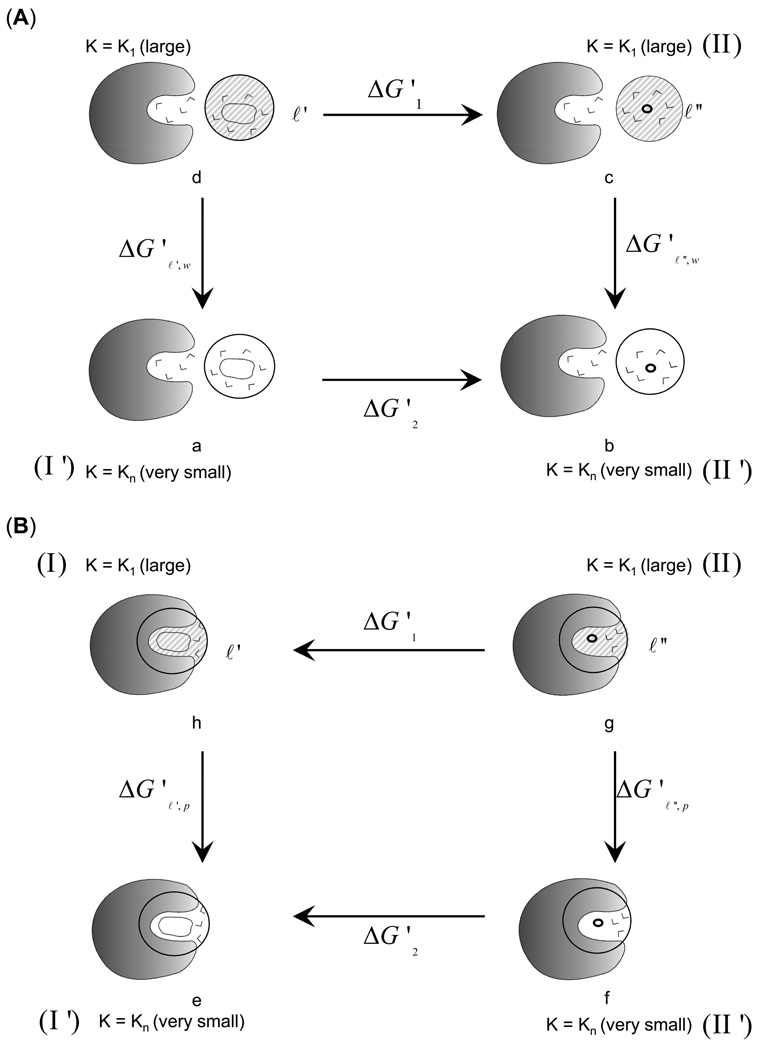
Figure 4.
The thermodynamics cycle used for the evaluation of the entropic contribution from hydrophobic effect. (A) The upper cycle (a→b→c→d) provides the hydrophobic contribution in water (). The cycle involves the release of the constraint for the non-polar-ligand (ℓ') and “nothing” (ℓ"), see text for details. (B) The lower cycle (e→f→g→h) provides the corresponding contribution in the protein (). The difference between the entropy values obtained from the two cycles provides the loss in entropy upon moving the non-polar ligand (ℓ') from water to protein ().