Table 3.
Scope of the [(Rp,S)-COP-Cl]2-Catalyzed Rearrangement of (E)-O-Allyl Carbamothioates
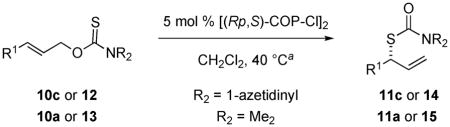 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R2 | time, hc | product |
||
| compd | yield %d | ee %e | ||||
| 1b | n-Pr | 1-azetinyl | 15 | ent-11c | 98 | 83 |
| 2b | n-Pr | Me2 | 18 | ent-11a | 72 | 82 |
| 3 | CH2CH(CH3)2 | 1-azetinyl | 42 | 14a | 85 | 80 |
| 4 | CH2OTBDMS | 1-azetinyl | 20 | 14b | 85 | 88 |
| 5 | CH2OTBDMS | Me2 | 18 | 15a | 97 | 87f,g |
| 6 | CH2OTIPS | 1-azetinyl | 42 | 14c | 99 | 87f |
| 7 | CH2OTBDPS | 1-azetinyl | 42 | 14d | 86 | 76f |
| 8 | CH2OH | 1-azetinyl | 13 | 14e | 55 | 61 |
| 9 | CH2NPh(Boc) | 1-azetinyl | 44 | 14f | 68 | 71 |
| 10 | CH2NPh(Boc) | Me2 | 43 | 15b | 77 | 81 |
| 11 | (CH2)2COMe | 1-azetinyl | 11 | 14g | 85 | 76 |
Substrate concentration = 0.5 M.
Sp,R enantiomer of [COP–Cl]2 was used.
Time for disappearance of starting material by TLC analysis.
Yield of product after purification by column chromatography.
Determined by SFC analysis using a chiral stationary phase; results from duplicate experiments agreed within ± 1%.
Determined after conversion of the product to alcohol 14e.
Determined by GC analysis using a chiral stationary phase; results from duplicate experiments agreed within ±2%.
