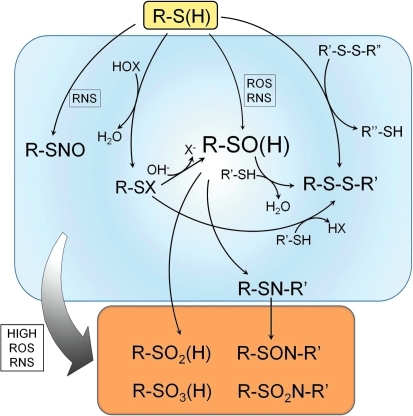
FIG. 1.
Biological modifications of cysteine thiols. Reactive cysteine thiols (R-SH), typically in their ionized, thiolate form (R-S−), are oxidized by hydrogen peroxide, organic hydroperoxides, hypohalous acids (HOX), and peroxynitrite to form sulfenic acids, which may be stabilized or go on to form other reversible (disulfides [R-SS-R′] or sulfenamides [R-SN-R′]) or irreversible oxidation products (sufinic acids [R-SO2H], sulfonic acids [R-SO3H], sulfinamides [R-SON-R′], and sulfonamides [R-SO2N-R′]). Reversible S-nitrosocysteine modifications are also observed in cells exposed to RNS. Both ROS and RNS promote these oxidations. Modifications within the blue box are considered reversible (by cellular reductants like thioredoxin and glutathione), whereas the species in the orange box are not. Although sulfinic and sulfonic acids are shown here as irreversible modifications, recent discoveries show that sulfinic acid forms of some Prxs can be recovered through action of specialized sulfinic acid reductases (sulfiredoxins). Prx, peroxiredoxin; RNS, reactive nitrogen species; ROS, reactive oxygen species. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).