Abstract
Death receptor targeting has emerged as one of the promising novel approaches of cancer therapy. The activation of one such prototypic death receptor, CD95 (Fas/APO-1), has remained controversial because CD95 agonistic molecules have exhibited either too strong toxicity or too little activity. The natural CD95 ligand (CD95L) is a cytokine, which needs to trimerize to mediate a cell death signal. Mega-Fas-Ligand, now referred to as APO010, is a synthetic hexameric CD95 agonist that exhibits strong antitumor activity in various tumor models. Here, we studied the effects of APO010 in human glioma models in vitro and in vivo. Compared with a cross-linked soluble CD95L or a CD95-agonistic antibody, APO010 exhibited superior activity in glioma cell lines expressing CD95 and triggered caspase-dependent cell death. APO010 reduced glioma cell viability in synergy when combined with temozolomide. The locoregional administration of APO010 induced glioma cell death in vivo and prolonged the survival of tumor-bearing mice. A further exploration of APO010 as a novel antiglioma agent is warranted.
Keywords: apoptosis, CD95 ligand, glioma, temozolomide
Death ligands of the tumor necrosis factor (TNF) family have been shown to induce apoptosis in a variety of human tumor models.1 The prototypic death ligand, CD95 ligand (CD95L), was identified as a natural ligand of receptor molecules previously recognized to be activated by cytolytic antibodies referred to as CH11 anti-Fas2 or anti-APO-13 in humans or Jo2 in mice.4 Agonistic antibodies to CD955,6 as well as CD95L7 were cytolytic for malignant glioma cells in vitro. In vivo applications were initially not further pursued because of hepatotoxicity, despite some preliminary evidence of the feasibility at least of a local application.8,9 CD95 is the principal receptor for CD95L, although a soluble decoy receptor, DcR3, has also been identified and may act to protect glioma cells from apoptosis.10
Given the toxicity associated with CD95 agonism, Apo2 ligand/TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) became the most promising death ligand for an application in the clinic, including malignant glioma.11–13 Apo2L/TRAIL interacts with at least 5 receptors: death receptor (DR)4/TRAIL-R1 and DR5/TRAIL-R2 transmit an apoptotic signal, whereas DcR1/TRAIL-R3 and DcR2/TRAIL-R4 do not. Further, osteoprotegerin is a low-affinity soluble receptor for Apo2L/TRAIL of unknown physiological significance.14 However, even Apo2L/TRAIL was not nontoxic to normal cells, and it became clear that efficacy and tolerability of Apo2L/TRAIL depend on specific modifications of the recombinant cytokine.15 A natural human Apo2L/TRAIL referred to as Apo2L.0 containing amino acids 114–281 was presumed to be well tolerated while retaining tumor activity.16 Current clinical trials are testing Apo2L in several solid tumors and non-Hodgkin lymphoma (ClinicalTrials.gov identifications NCT00671372, NCT00873756, NCT00400764, NCT00923390, NCT00819169, and NCT00508625).
Both CD95L and Apo2L/TRAIL recruit Fas-associated death domain protein to their death-promoting receptors and consecutively trigger caspase-mediated cell death. In the ongoing search for a potent death ligand with an acceptable safety profile, Holler et al.17 developed the “Mega-Fas-Ligand,” a hexameric protein consisting of 2 CD95L extracellular domain trimers and the collagen domain of adiponectin ACRP30. This molecule is now known as APO010. Here, we characterize the cytolytic properties of APO010 on human glioma cells in vitro and in vivo and compare its potency to a cross-linked soluble CD95L and an agonistic anti-CD95 antibody.
Materials and Methods
Materials and Cell Lines
The human malignant glioma cell lines LN-308, LNT-229 (T for Tübingen for clarification), and U87MG were provided by Dr. N. de Tribolet (Centre Hospitalier Universitaire Vaudois). The simian virus 40–fetal human astrocytic cell line (SV-FHAS) was provided by D. Stanimirovic (Institute of Biological Sciences, National Research Council of Canada). The generation of LNT-229.neo control and LNT-229.MGMT transfectants overexpressing O6-methylguanine-DNA methyltransferase (MGMT) has been described,18 as well as the generation of LNT-229 cells overexpressing crm-A.19 To stably overexpress CD95, LN-308 cells were transfected with the BCMGS.neo expression vector containing the cDNA for CD95 using Metafectene Pro. The generation of the expression vector has been described.6 The LN-308.CD95 transfectants and the LN-308.neo control cells were selected with G418 (500 µg/mL), and LN-308.CD95 cells were further subcloned. Primary glioblastoma cells were established from freshly resected tumors, cultured in monolayers, and used between passages 4 and 9.20 Cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum (FCS), 2 mM glutamine, and penicillin (100 IU/mL)/streptomycin (100 mg/mL).
DEVD-amc and zVAD-fmk were obtained from Bachem. Propidium iodide (PI) was purchased from Sigma. Temozolomide (TMZ) was obtained from Schering Plough. APO010 was provided by Topotarget.17 O6-Benzylguanine (O6BG) was a gift from Bernd Kaina.21 sCD95L and enhancer were obtained from Alexis. sCD95L consists of the extracellular domain of human CD95L (amino acids 103–281) fused at the N-terminus to a linker peptide (26 amino acids) and a FLAG-tag. The cross-linking enhancer increases the biological activity by ∼50-fold. sCD95L and enhancer were used at a ratio of 1:5. In the following, the term sCD95L stands for the combination of sCD95L and the enhancer molecule. An agonistic antibody to CD95 (clone CH11) was from Upstate.2
RNA Silencing
To silence endogenous CD95 expression, U87MG and LNT-229 cells were transiently transfected with 50 nM HS_FAS_7 HP validated small-interfering RNA (siRNA) targeting CD95 from Qiagen (Cat No. SI02654463; sense strand 5′-GGAGUACACAGACAAAGCCTT-3′). All Stars nonsilencing siRNA from Qiagen (Cat No. 1027280) was used as a negative control. Glioma cells were seeded in 24-well plates and 24 hours later transfected with siRNA using Metafectene Pro. The extent of gene silencing was verified by the analysis of CD95 expression on the cell surface by flow cytometry.
DEVD-amc Cleavage Assay
The cells were seeded in 96-well plates, treated as indicated, lysed in 25 mM Tris–HCl, pH 8.0, 60 mM NaCl, 2.5 mM EDTA, 0.25% Nonidet-P40 for 10 minutes, and DEVD-amc was added at 12.5 mM. Caspase activity was assessed by fluorescence using a Berthold Mithras fluorimeter (Berthold Technologies) at 355 nm excitation and 475 nm emission wavelengths.
Flow Cytometry for CD95 Expression
Adherent glioma cells were detached using Accutase (PAA Laboratories) and blocked with 2% FCS in phosphate-buffered saline (PBS). The cells were incubated for 30 minutes on ice using the fluorescein isothiocyanate (FITC)–antihuman CD95 monoclonal antibody (clone UB2) or immunoglobulin (Ig) G1 isotype control from Beckman Coulter. Flow cytometry was performed with a Dako flow cytometer (Dako). Signal intensity was calculated by dividing median fluorescence obtained with the specific antibody by signal intensity obtained with the isotype control antibody (specific fluorescence index, SFI).
Detection of Apoptosis by Annexin V Binding
Apoptotic cell death was analyzed by staining with FITC-labeled annexin V (BD Bioscience). Glioma cells were treated as indicated, collected, washed with PBS, and resuspended in a buffer containing 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)/NaOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2. Then annexin V–FITC and PI were added. After incubation for 30 minutes, the cells were analyzed by flow cytometry (Dako CyAn ADP 7). Cells positive for annexin V binding and negative for PI staining were considered as early apoptotic. Cells positive for annexin V binding and positive for PI staining were considered as late apoptotic cells.
Growth and Viability Assay
The cells were seeded in 96-well plates and allowed to attach for 24 hours. Cells were treated as indicated, and cell density of attached cells was assessed by crystal violet staining. Briefly, the cell culture medium was removed and surviving cells were stained with 0.5% crystal violet in 20% methanol for 20 minutes at room temperature. The plates were washed extensively under running tap water and air-dried, and optical density values were read in an ELISA reader (Mithras LB 940, Berthold Technologies) at a wavelength of 550 nm.
Immunoblot Analysis
The general procedure has been described.22 The cells were treated as indicated and lysed. Twenty micrograms of protein per lane were separated on 10%–12% acrylamide gels (Bio-Rad). After transfer to a nitrocellulose membrane, the blots were pretreated for 1 hour with PBS containing 5% skim milk and 0.05% Tween 20 and then incubated overnight with the following antibodies: cleaved caspase 3 (No. 9661) from Cell Signaling, caspase 8 (ALX-804-429-C100) from Alexis, poly(ADP-ribose) polymerase (PARP; 4C10-5) from BD Bioscience, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from Chemicon. Visualization of protein bands was accomplished using horseradish peroxidase–coupled IgG secondary antibody (Santa Cruz) and enhanced chemoluminescence (Amersham).
Animal Experiments
CD1nu/nu mice were purchased from Charles River Laboratories. The experiments were performed according to the German animal protection law. For the intracranial glioma model, mice aged 6–12 weeks were anesthetized and placed in a stereotaxic fixation device (Stoelting). A burr hole was drilled in the skull 2 mm lateral to the bregma. The needle of a Hamilton syringe was introduced to a depth of 3 mm. U87MG cells (105) resuspended in a volume of 2 µL of PBS were injected into the right striatum. Locoregional treatment with APO010 (40 ng in 2 µL of PBS on days 7 and 14 after tumor implantation) or PBS was performed similarly (6 mice per group). The mice employed in our experiments had body weights between 20 and 30 g. Thus, the mice received 0.0013–0.002 mg/kg body weight APO010 i.c. Systemic treatment was performed by i.p. injections of APO010 (0.015 mg/kg body weight 3 times per week) or PBS (5 mice per group). These doses were selected based on a maximum tolerated dose (MTD) for systemic application according to Verbrugge et al.23 and by an MTD determination for i.c. application performed in the context of this study. The mice were observed twice daily and killed when developing neurological symptoms or at defined time points for histological analysis as indicated. For the detection of intratumoral APO010, U87MG tumors were inoculated and the mice were treated i.c. or i.p. as described above on day 14 following tumor inoculation. Mice were sacrificed 4 (n = 3) or 24 hours (n = 3), respectively, following treatment. Mice were cardially perfused with cold PBS and brains were snap frozen. The tumors were explanted, homogenized, and brought into lysis buffer containing a protease inhibitor cocktail. Lysates were centrifuged at 6000 turns/min for 5 minutes, and the supernatant extracted for gel electrophoresis and immunoblot using anti-ACRP30 monoclonal antibody (ALX-804-144, clone ne.na) from Enzo Life Sciences, which recognizes the collagen domain of adiponectin ACRP30 as part of APO010.
Immunohistochemistry
For histology, 8 mm cryostat sections were stained with hematoxylin-eosin. Cleaved caspase 3 (AF835; R&D Systems) was detected by immunohistochemistry. The tissue sections were pretreated with 3% H2O2 in methanol to block endogenous peroxidase and boiled for 10 minutes in citrate buffer. Endogenous biotin activity was blocked with the Dako biotin blocking kit (DakoCytomation GmbH). Sections were blocked with 3% skim milk and 10% normal swine serum for 30 minutes each at room temperature. Primary antibodies were added and the tissue sections were incubated overnight at 4°C in a humidified chamber. Purified normal IgG substituted for the specific antibody served as a negative control. The sections were washed 3 times with PBS containing 0.05% Tween 20 (PBST). Biotinylated goat antirabbit secondary antibody (Vector Laboratories) was added for 1 hour. After 3 PBST washes, streptavidin peroxidase conjugate (Zymed) was added, and the tissue sections were incubated for 10 minutes, washed with PBST, and 3-amino-9-ethylcarbazole in N,N-dimethylformamide (Zymed) was added as a substrate. After a further 10 minutes of incubation, the sections were finally washed with water. Terminal deoxynucleotidyl transferase mediated X-dUTP nick end labeling (TUNEL) was performed using the TUNEL-AP Kit (Roche). Neighboring control sections were stained using Mayer's hematoxylin. All sections were coverslipped, and staining was assessed by light microscopy.
Statistics
Statistical significance was assessed by one-way analysis of variance (ANOVA) between groups and Bonferroni post hoc testing or Student 2-tailed t-test as indicated (SPSS 17, SPSS). Synergy of APO010 and TMZ was evaluated using the fractional product method. Here, “predicted values” correspond to an additive action of 2 compounds that can be compared with the observed effect.24
Results
APO010 Is Cytotoxic to Human Glioma Cells
The human glioma cell lines studied here express different levels of CD95 as assessed by flow cytometry using an antihuman CD95-specific antibody (clone UB2). The SFIs for the present culture conditions were 2.3 for U87MG, 2.1 for LNT-229, and 1.3 for LN-308. The SFI for SV-FHAS was 1.1.
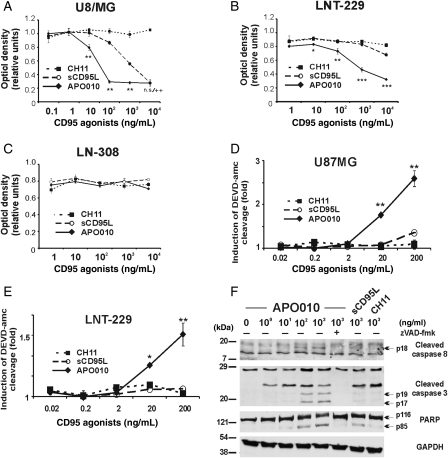
The lower level of CD95 expression has been held responsible for the relative resistance of LN-308 cells to CD95-mediated apoptosis.6 Accordingly, U87MG and LNT-229, but not LN-308, cells were highly sensitive to APO010. At equivalent concentrations, APO010 was more effective than sCD95L or CH11 in inducing cell death in both cell lines (Fig. 1A–C). Accordingly, APO010 induced DEVD-amc–cleaving caspase activity in U87MG and LNT-229 cells (Fig. 1D and E) but not in LN-308 cells (data not shown). Cell death induction involved the cleavage of caspases 8 and 3 and of PARP, and caspase and PARP processing were blocked by the broad-spectrum caspase inhibitor zVAD-fmk (Fig. 1F). The induction of cell death by APO010 critically depended on the activation of caspase 8, since LNT-229 cells overexpressing crm-A, which selectively blocks caspase 8, were resistant (data not shown).
Fig. 1.
APO010 induces DEVD-amc–cleaving caspase activity and cell death in human glioma cell lines. (A–C) U87MG, LNT-229, or LN-308 cells were exposed to increasing concentrations of APO010, sCD95L, or CH11 for 20 hours in triplicates. Cell density was assessed by crystal violet staining. Data are expressed as mean and standard deviation (n = 3); 1 representative out of 3 independent experiments with similar results is shown. (D and E) U87MG or LNT-229 cells were treated as in (A) and (B) in triplicates and assessed at 3 (U87MG) or 6 hours (LNT-229) for DEVD-cleaving caspase activity. Data are expressed in relation to untreated controls as mean and standard deviation (n = 3); 1 representative out of the 3 independent experiments with similar results is shown. The level of statistical significance for a superior effect of APO010 over sCD95L and CH11 was assessed in (A)–(E) by one-way ANOVA and Bonferroni post hoc testing (*P < .05; **P < .01; ***P < .001; ++P < .01 for APO010 and sCD95L vs CH11). (F) LNT-229 cells were treated as indicated for 20 hours. Cellular lysates were examined for cleavage of caspases 8 and 3 and for PARP and GAPDH by immunoblot.
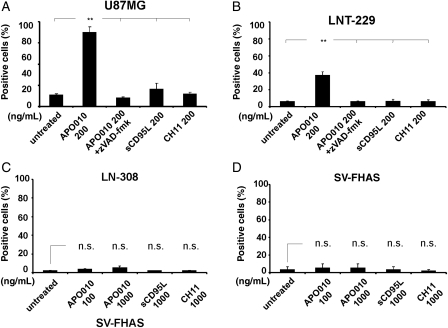
Cell death induction by APO010 was further characterized by flow cytometry using annexin V labeling for the assessment of apoptosis and PI staining for the detection of cell death. APO010 was more effective in inducing apoptosis than sCD95L or CH11, and the induction of cell death was blocked by zVAD-fmk. Consistent with the cell loss depicted in Fig. 1, U87MG and LNT-229 cells were sensitive (Fig. 2A and B), whereas LN-308 cells were resistant (Fig. 2C). Moreover, SV-FHAS astrocytes were resistant to APO010 (Fig. 2D).
Fig. 2.
Caspase-dependent induction of annexin V/PI labeling by APO010. The cells were treated with CD95 agonists as indicated and characterized by annexin V/PI labeling 6 (U87MG) or 20 hours (LNT-229) later. Quantitative data for U87MG, LNT-229, LN-308, and SV-FHAS cells are provided in (A)–(D). Coexposure to zVAD-fmk (50 μM) was included to assess the caspase dependency of cell death induction. Data are expressed as the mean percentage of annexin V–positive cells ± SEM from the 3 independent experiments. The level of statistical significance was assessed by one-way ANOVA and Bonferroni post hoc testing (**P < .01).
Further, we tested primary glioma cell cultures from 9 randomly selected glioblastoma patients. These ex vivo propagated cells were treated between passages 4 and 9 with either APO010, sCD95L, or CH11. Again, APO010 was more effective in inducing apoptosis than sCD95L or CH11 as assessed by annexin V/PI labeling and flow cytometry (Table 1). APO010 killed in a concentration-dependent manner more than 50% of the cells at a concentration of 1000 ng/mL in 7 tumors, compared with 3 tumors for sCD95L and none of the tumors for CH11 at equivalent concentrations. Only 2 (TU 132 and TU 159) of these 9 tested tumors showed relative resistance to APO010-mediated cell death. TU 132 does not express CD95 on the cell surface (SFI 1.0) as assessed by flow cytometry and TU 159 only at a low level (SFI 1.4; Table 1). Most of the sensitive tumors show higher SFI values for CD95 expression. However, the SFI of the sensitive TU 447 (SFI 1.4) corresponded to that of TU 159, indicating the regulation of sensitivity at the level of downstream signal transduction too.
Table 1.
APO010 induces apoptotic cell death in ex vivo human glioma cell cultures
| SFI | Apoptotic cells [%] ± SEM |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| APO010 |
APO010 + zVAD-fmk (1000 ng/mL) | sCD95L (1000 ng/mL) | CH11 (1000 ng/mL) | ||||||
| 0 ng/mL | 1 ng/mL | 10 ng/mL | 100 ng/mL | 1000 ng/mL | |||||
| TU 113 | 2.8 | 4 ± 0.4 | 10 ± 4.2 | 67 ± 7.8 | 89 ± 1.8 | 92 ± 2.8 | 7 ± 2.1 | 77 ± 4.2 | 11 ± 1.4 |
| TU 159 | 1.4 | 7 ± 0.8 | 7 ± 1.2 | 11 ± 2.4 | 19 ± 3.1 | 21 ± 2.2 | 5 ± 1 | 9 ± 2.4 | 7 ± 0.7 |
| TU 408 | 1.7 | 7 ± 0.7 | 12 ± 2.5 | 18 ± 0.9 | 45 ± 6.1 | 52 ± 5.7 | 9 ± 1.2 | 28 ± 0.3 | 10 ± 0.3 |
| TU 409 | 1.6 | 9 ± 1.7 | 9 ± 1.4 | 30 ± 6.8 | 61 ± 10.2 | 69 ± 7.5 | 8 ± 0.3 | 41 ± 0.3 | 9 ± 0.9 |
| TU 426 | n.d. | 10 ± 2.4 | 19 ± 4.9 | 49 ± 13.6 | 63 ± 9.6 | 69 ± 9.2 | 9 ± 1.9 | 28 ± 4.1 | 15 ± 2.9 |
| TU 431 | 2.5 | 8 ± 3.5 | 6 ± n.d. | 26 ± n.d. | 64 ± 1.8 | 73 ± 2.1 | 7 ± 1.4 | 34 ± 1.4 | 13 ± 4.9 |
| TU 447 | 1.4 | 10 ± 0.4 | 24 ± 3.2 | 63 ± 1.8 | 69 ± 3.2 | 69 ± 4.2 | 13 ± 3.5 | 53 ± 3.9 | 29 ± 6.7 |
| TU 132 | 1 | 3 ± n.d. | 4 ± n.d. | 4 ± n.d. | 3 ± n.d. | 2 ± n.d. | 3 ± n.d. | 3 ± n.d. | 3 ± n.d. |
| TU 446 | n.d. | 2 ± n.d. | n.d. | n.d. | 75 ± n.d. | 79 ± n.d. | 2 ± n.d. | 57 ± n.d. | 9 ± n.d. |
Cell cultures were generated from freshly resected tumor specimens from 9 randomly selected glioblastoma patients. Cells were treated between passages 4 and 9 as indicated for 20 hours. Apoptosis was assessed by annexin V/PI labeling and flow cytometry. Data are expressed as mean percentage of annexin V positive cells ± SEM (n = 1 − 3; n.d., not defined). CD95 expression was assessed by flow cytometry and SFI values are indicated.
Cytotoxicity of APO010 Critically Depends on the Expression of CD95
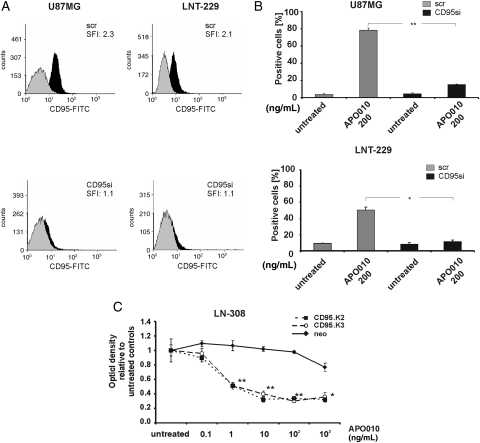
To assess the specificity of the cytotoxic activity of APO010, CD95 expression was silenced using siRNA in LNT-229 and U87MG glioma cells. Successful gene silencing was verified by flow cytometry 72 hours following transfection (Fig. 3A). Time-course experiments confirmed a stable knockdown of CD95 between 72 and 96 hours following transfection (data not shown). The knockdown of CD95 led to a significant reduction in sensitivity of both cell lines toward APO010-mediated cytotoxicity (Fig. 3B). To sensitize LN-308 cells toward APO010, these cells were genetically modified to overexpress CD95 and subcloned. The clones LN308.CD95 K2 and K3 showed stable overexpression of CD95 as assessed by flow cytometry (SFIs 7.0 and 14.4) and gained sensitivity toward APO010 (Fig. 3C).
Fig. 3.
Targeted alterations in CD95 expression at the cell surface result in altered sensitivity toward APO010. U87MG or LNT-229 cells were transfected with siRNA targeting CD95 (CD95si) or scrambled control siRNA (scr). (A) Seventy-two hours after transfection, the cells were assessed for the expression of CD95 by flow cytometry. (B) Seventy-two hours after transfection the cells were treated with APO010 (200 ng/mL) and characterized by annexin V/PI labeling 6 (U87MG) or 20 hours (LNT-229) later. Data are expressed as the mean percentages of annexin V–positive cells ± SEM from the 3 independent experiments. (C) LN-308 cells were stably transfected to overexpress CD95. Subclones LN-308.CD95 K2 or K3 and LN-308.neo controls were exposed to increasing concentrations of APO010 for 20 hours in triplicates in a 96-well format. Cell density was assessed by crystal violet staining. Data are expressed in relation to untreated controls as mean and SEM (n = 3). One of the 3 representative independent experiments with similar results is shown. The level of statistical significance was assessed by one-way ANOVA and Bonferroni post hoc testing (*P < .05; **P < .01).
APO010 Synergizes with TMZ in Inducing Glioma Cell Death
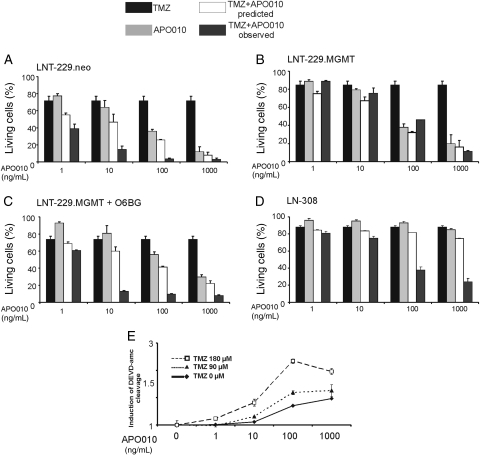
The next set of experiments was performed to evaluate additive or synergistic effects of APO010 and TMZ, the most commonly used drug for the treatment of glioblastoma. According to the fractional product method,24 the combination of APO010 and TMZ had more than additive, that is, synergistic, cytotoxic effects (Fig. 4A). Resistance to TMZ alone is conferred by the DNA repair enzyme MGMT.18 To assess whether the combination of APO010 and TMZ bypassed the protective function of MGMT, we cotreated MGMT-transfected cells with APO010 and TMZ. No synergy was observed under these conditions (Fig. 4B), suggesting that the MGMT-sensitive effects of TMZ mediate synergy. Accordingly, when higher, equieffective concentrations for monotherapy with TMZ were employed in the MGMT-transfected cells, synergy was restored (data not shown). Moreover, pre-exposure of the MGMT-transfected cells to the MGMT inhibitor O6BG and subsequent coexposure to lower concentrations of TMZ and APO010 also resulted in synergistic induction of cell loss (Fig. 4C). These data corroborated that no specific, MGMT-independent cascade triggered by TMZ facilitates APO010-induced apoptosis. Importantly, LN-308 cells, which are resistant to death receptor–mediated apoptosis, were sensitized by TMZ to APO010-mediated cell death (Fig. 4D). However, since LN-308 cells are also relatively resistant to TMZ-mediated cell death,18 a higher concentration of TMZ compared with LNT-229 cells was necessary to achieve this sensitization. Moreover, the synergy of TMZ and APO010 led to enhanced DEVD-amc–cleaving caspase activity in LNT-229 cells (Fig. 4E).
Fig. 4.
Synergistic induction of cell death by APO010 and TMZ. LNT-229 cells were treated with TMZ for 96 hours followed by APO010 for another 20 hours. The graphs show the results of treatment with either agent alone, the predicted effect assuming independent (additive) effects, and the truly observed effect. The bars express the percentage of living cells as assessed by flow cytometry after annexin V and PI staining. The data are expressed as mean and SEM from 3 independent experiments. We studied LNT-229.neo control cells (TMZ 90 μM) (A), MGMT-transfected cells (TMZ 90 μM) (B), MGMT-transfected cells additionally treated with O6BG (50 µM; TMZ 90 μM) (C), and LN-308 cells (TMZ 600 μM) (D). (E) LNT-229 cells were pretreated with TMZ (90 μM) as indicated above followed by addition of APO010 and assessed at 6 hours for DEVD-amc–cleaving caspase activity. Data are expressed relative to untreated controls as mean and standard deviation (n = 3). One representative out of the 3 independent experiments with similar results is shown.
APO010 Induces Glioma Cell Death In Vivo and Prolongs Survival
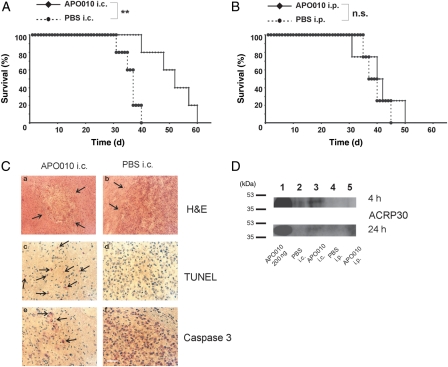
Orthotopic human U87MG xenografts were established in nude mice as outlined in the “Materials and Methods” section and were confirmed to be associated with intracranial tumor growth as assessed by histology. APO010 was administered by stereotactic injection on days 7 and 14 after tumor cell implantation. The dose of 40 ng per single injection was determined from tolerability studies in preceding applications in non-tumor-bearing mice. APO010 conferred a significant survival benefit. At 40 days after tumor cell implantation, the survival rate was 80% as opposed to 0% in control-treated animals (P < .01; Student 2-tailed t-test). The median survival increased from 37 to 52 days (Fig. 5A). The subsequent death of the mice in the APO010 treatment group was related to neurological symptoms following recurrence of tumor growth as assessed macroscopically.
Fig. 5.
Locoregional APO010 administration induces glioma cell death in vivo and prolongs the survival of tumor-bearing mice. (A) The mice were treated i.c. with PBS or APO010 (40 ng) on days 7 and 14 after the intracranial implantation of 105 U87MG cells and monitored for survival. (B) The mice were treated with PBS or APO010 (0.015 mg/kg body weight) i.p. 3 times per week and monitored for survival. (C) The animals were treated as in (A) and tumors were removed on day 15 after tumor inoculation and stained with hematoxylin-eosin (a and b) or for apoptotic cell death by TUNEL (c and d) or immunohistochemistry for activated caspase 3 (e and f). Size bar in the lower right panel: 40 µm. Analysis of statistical significance was performed by Student 2-tailed t-test (**P < .01; n.s., not significant). (D) Mice were treated as described in (A) and (B) with a single injection. After perfusion of the mice with PBS, the tumors were removed 4 (upper panel) or 24 hours (lower panel) after treatment. Pooled tumor lysates were subjected to immunoblot, and APO010 was assessed using an anti-ACRP30 antibody (clone ne.na). Purified APO010 served as a positive control.
Systemic treatment was performed by i.p. injections of APO010 with the MTD of 0.015 mg/kg body weight 3 times per week following tumor inoculation until the mice had to be sacrificed due to the development of neurological symptoms. The survival of the mice in the treatment and PBS control groups did not differ significantly (P = n.s.; Student 2-tailed t-test; Fig. 5B).
Histological studies were performed on day 15 after tumor inoculation in i.c.-treated mice. Hematoxylin-eosin staining revealed minimal tumor burden in the APO010 group compared with controls (Fig. 5Ca and b). Apoptotic tumor cells were detected by TUNEL staining (Fig. 5Cc and d) and immunohistochemistry for active caspase 3 (Fig. 5Ce and f) only in the APO010 group but not the control group (Fig. 5C middle and lower panels).
The failure of systemic application to limit tumor growth suggested insufficient APO010 delivery to the target. Accordingly, to assess intratumoral APO010, tumors were implanted and 6 mice per group were treated by a single i.p. or i.c. administration of APO010 or PBS as a control. Three mice per group were sacrificed 4 hours and 3 mice 24 hours following treatment. Tumor lysates were assessed for APO010 by immunoblot. APO010 was detected in only the tumors from mice injected i.c. with APO010, but not in the APO010 i.p. or the PBS control groups (Fig. 5D). At 24 hours (Fig. 5D, lower panel), note a weak band in lane 3 and only background signal in lane 5. Equal protein loading was ascertained by Ponceau S staining (data not shown).
Discussion
This article reports on a further development in the field of death receptor targeting for malignant glioma. We found that APO010, a novel hexameric CD95 agonist,17 is superior to either soluble CD95L or the agonistic CD95 antibody CH11 in killing CD95-expressing U87MG and LNT-229 glioma cells (Figs 1 and 2). Superior activity of APO010 over other CD95 agonists has previously been observed in leukemia and SKOV-3 ovarian cancer cells.25,26 The induction of cell death in glioma cells was specific in that it was receptor mediated. In LN-308 cells, which express very little CD95, a significant induction of cell death was observed for only the highest doses of APO010 (Figs 1C and 2C), and LNT-229 cells overexpressing crm-A were resistant to APO010 (data not shown). Moreover, in contrast to human Apo2L/TRAIL, which failed to kill freshly isolated glioma cells,27 activity of APO010 was also observed in various primary glioma cell cultures (Table 1). The CD95 gene silencing in LNT-229 or U87MG cells abrogated, and the overexpression of CD95 in LN-308 cells conferred sensitivity of these cells (Fig. 3). Therefore, the cell surface expression of CD95 is a prerequisite for the cytotoxic action of APO010, and the expression level of CD95 is a gross indicator for the sensitivity. However, the susceptibility of the cell lines is likely modulated additionally by downstream intracellular targets, such as caspases and inhibitors of apoptosis proteins (IAPs). Compared with LNT-229 cells, U87MG cells express similar levels of X-linked IAP28 but much higher levels of caspase 9,29 suggesting that these cells amplify the mitochondrial cell death pathway more efficiently. Such observations explain that cell lines expressing similar amounts of CD95 may show different sensitivities.
Additive or synergistic effects of death ligands and chemotherapy have been studied extensively in various cancer models, including malignant gliomas.7,13,30 APO010 exhibited enhanced activity when combined with platinum in ovarian cancer cells25 or imatinib in gastrointestinal stromal tumors.31 In contrast, no synergistic effect of APO010 was demonstrated in vivo when combined with irradiation.23 In view of a possible clinical application, we combined APO010 with TMZ, the current standard of care chemotherapy for glioblastoma.32 There was compelling evidence for synergy as assessed by the fractional product method, and this synergy required the classical MGMT-sensitive cell death pathway triggered by TMZ (Fig. 4). Sensitization of glioma cells toward Apo2L/TRAIL-mediated apoptosis by lomustine, another alkylating chemotherapeutic agent, involved the mitochondrial pathway and enhanced cytochrome c release.30 Another report demonstrated an upregulation of CD95 following treatment of U87MG cells with the O6-methylating agent N-methyl-N-nitro-N-nitrosoguanide by immunoblot in membrane extracts.33 In this study, we did not observe an upregulation of CD95 on the cell surface by flow cytometry under conditions of synergistic killing in LN-308 cells (data not shown).
Local injection of APO010 conferred a significant survival benefit to glioma-bearing mice in the absence of relevant toxicity (Fig. 5). APO010 was active in vivo only when administered locally but not when given i.p. Assessment of tumors ex vivo revealed APO010 in only the i.c.-treated group but not following i.p. injection (Fig. 5D). This suggests that upon systemic treatment with the MTD, too little, if any, APO010 reaches the tumor. In general, the toxicity of the human APO010 in mice in vivo has been favorable compared with the mouse Jo2 antibody. When applied in BALB/c mice, 100 µg of Jo2 killed most of the mice and 10 µg killed half of the mice when given i.p.4 In contrast, APO010 has been administered systemically23 or i.p.25 or intralesionally in the brain in this study and controlled tumor growth in the absence of unacceptable toxicity as assessed by the clinical observation of the mice and immunohistochemistry. The MTD for APO010 was 0.015 mg/kg body weight for systemic treatment23 and 40 ng per single injection for local intracerebral treatment as determined here. Kamei et al.34 used the anti-CD95 antibody, clone CH11, in a mouse model of melanoma and injected 5 µg of the antibody directly in the tumors grown s.c. in nude mice. This was tolerated but did not affect tumor growth.34 The injection of up to 10 µg of CH11 into s.c. growing tumors derived from SNB19 or SNB79 glioblastoma cell lines was tolerated too but did not alter tumor growth either.35 In the same study, mice were also injected with up to 10 µg of CH11 i.v. An MTD was not formally determined in either study.
The MTDs of APO010 (40 ng) and CH11 (10 µg) for local treatment are difficult to compare, since APO010 was used in an orthotopic model and injected i.c., whereas CH11 was tested only in s.c. inoculated tumors. No study available has directly compared APO010 with an anti-CD95 antibody or a soluble Fas ligand in vivo. However, in the present study, APO010 exhibited antiglioma activity when injected directly in the tumor, whereas CH11 failed to exhibit activity when injected s.c. in mouse models of melanoma or glioblastoma as indicated above.
APO010 is now being explored in a phase I dose-escalation study in up to 35 patients with untreatable advanced or refractory solid tumors in order to establish the safety and tolerability and MTD in humans (ClinicalTrials.gov identification NCT00437736). In addition, pharmacokinetics, immunogenicity, and preliminary signs of anticancer activity are being evaluated.
Conflict of interest statement. None declared.
Funding
M.W. acknowledges support by Swiss National Fund (NCCR Neuro).
References
- 1.Papenfuss K, Cordier SM, Walczak H. Death receptors as targets for anti-cancer therapy. J Cell Mol Med. 2008;12:2566–2585. doi: 10.1111/j.1582-4934.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trauth BC, Klas C, Peters AM, et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 4.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 5.Weller M, Frei K, Groscurth P, et al. Anti-Fas/APO-1 antibody-mediated apoptosis of cultured human glioma cells. Induction and modulation of sensitivity by cytokines. J Clin Invest. 1994;94:954–964. doi: 10.1172/JCI117462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller M, Malipiero U, Rensing-Ehl A, Barr PJ, Fontana A. Fas/APO-1 gene transfer for human malignant glioma. Cancer Res. 1995;55:2936–2944. [PubMed] [Google Scholar]
- 7.Roth W, Fontana A, Trepel M, et al. Immunochemotherapy of malignant glioma: synergistic activity of CD95 ligand and chemotherapeutics. Cancer Immunol Immunother. 1997;44:55–63. doi: 10.1007/s002620050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rensing-Ehl A, Frei K, Flury R, et al. Local Fas/APO-1 (CD95) ligand-mediated tumor cell killing in vivo. Eur J Immunol. 1995;25:2253–2258. doi: 10.1002/eji.1830250821. [DOI] [PubMed] [Google Scholar]
- 9.Ambar BB, Frei K, Malipiero U, et al. Treatment of experimental glioma by administration of adenoviral vectors expressing Fas ligand. Hum Gene Ther. 1999;10:1641–1648. doi: 10.1089/10430349950017644. [DOI] [PubMed] [Google Scholar]
- 10.Roth W, Isenmann S, Nakamura M, et al. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759–2765. [PubMed] [Google Scholar]
- 11.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieger J, Naumann U, Glaser T, Ashkenazi A, Weller M. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 1998;427:124–128. doi: 10.1016/s0014-5793(98)00409-8. [DOI] [PubMed] [Google Scholar]
- 13.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 14.Zauli G, Melloni E, Capitani S, Secchiero P. Role of full-length osteoprotegerin in tumor cell biology. Cell Mol Life Sci. 2009;66:841–851. doi: 10.1007/s00018-008-8536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitsch R, Bechmann I, Deisz RA, et al. Human brain-cell death induced by tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL) Lancet. 2000;356:827–828. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence D, Shahrokh Z, Marsters S, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 17.Holler N, Tardivel A, Kovacsovics-Bankowski M, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermisson M, Klumpp A, Wick W, et al. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem. 2006;96:766–776. doi: 10.1111/j.1471-4159.2005.03583.x. [DOI] [PubMed] [Google Scholar]
- 19.Glaser T, Castro MG, Lowenstein PR, Weller M. Death receptor-independent cytochrome c release and caspase activation mediate thymidine kinase plus ganciclovir-mediated cytotoxicity in LN-18 and LN-229 human malignant glioma cells. Gene Ther. 2001;8:469–476. doi: 10.1038/sj.gt.3301415. [DOI] [PubMed] [Google Scholar]
- 20.Rieger J, Wick W, Weller M. Human malignant glioma cells express semaphorins and their receptors, neuropilins and plexins. Glia. 2003;42:379–389. doi: 10.1002/glia.10210. [DOI] [PubMed] [Google Scholar]
- 21.Kaina B, Muhlhausen U, Piee-Staffa A, et al. Inhibition of O6-methylguanine-DNA methyltransferase by glucose-conjugated inhibitors: comparison with nonconjugated inhibitors and effect on fotemustine and temozolomide-induced cell death. J Pharmacol Exp Ther. 2004;311:585–593. doi: 10.1124/jpet.104.071316. [DOI] [PubMed] [Google Scholar]
- 22.Eisele G, Wischhusen J, Mittelbronn M, et al. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129:2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 23.Verbrugge I, Wissink EH, Rooswinkel RW, et al. Combining radiotherapy with APO010 in cancer treatment. Clin Cancer Res. 2009;15:2031–2038. doi: 10.1158/1078-0432.CCR-08-2125. [DOI] [PubMed] [Google Scholar]
- 24.Webb J. Effects of more than one inhibitor. In: Webb J, editor. Enzymes and Metabolic Inhibitors. New York: Academic Press; 1963. pp. 487–512. [Google Scholar]
- 25.Etter AL, Bassi I, Germain S, et al. The combination of chemotherapy and intraperitoneal MegaFas Ligand improves treatment of ovarian carcinoma. Gynecol Oncol. 2007;107:14–21. doi: 10.1016/j.ygyno.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 26.Greaney P, Nahimana A, Lagopoulos L, et al. A Fas agonist induces high levels of apoptosis in haematological malignancies. Leuk Res. 2006;30:415–426. doi: 10.1016/j.leukres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Rieger J, Frank B, Weller M, Wick W. Mechanisms of resistance of human glioma cells to Apo2 ligand/TNF-related apoptosis-inducing ligand. Cell Physiol Biochem. 2007;20:23–34. doi: 10.1159/000104150. [DOI] [PubMed] [Google Scholar]
- 28.Wagenknecht B, Glaser T, Naumann U, et al. Expression and biological activity of X-linked inhibitor of apoptosis (XIAP) in human malignant glioma. Cell Death Differ. 1999;6:370–376. doi: 10.1038/sj.cdd.4400503. [DOI] [PubMed] [Google Scholar]
- 29.Glaser T, Weller M. Caspase-dependent chemotherapy-induced death of glioma cells requires mitochondrial cytochrome c release. Biochem Biophys Res Commun. 2001;281:322–327. doi: 10.1006/bbrc.2001.4349. [DOI] [PubMed] [Google Scholar]
- 30.Rohn TA, Wagenknecht B, Roth W, et al. CCNU-dependent potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells is p53-independent but may involve enhanced cytochrome c release. Oncogene. 2001;20:4128–4137. doi: 10.1038/sj.onc.1204534. [DOI] [PubMed] [Google Scholar]
- 31.Rikhof B, van der Graaf WT, Meijer C, et al. Abundant Fas expression by gastrointestinal stromal tumours may serve as a therapeutic target for MegaFasL. Br J Cancer. 2008;99:1600–1606. doi: 10.1038/sj.bjc.6604736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 33.Roos WP, Batista LF, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 34.Kamei T, Inui M, Nakase M, et al. Experimental therapy using interferon-gamma and anti-Fas antibody against oral malignant melanoma cells. Melanoma Res. 2005;15:393–400. doi: 10.1097/00008390-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Decaudin D, Beurdeley-Thomas A, Nemati F, et al. Distinct experimental efficacy of anti-Fas/APO-1/CD95 receptor antibody in human tumors. Exp Cell Res. 2001;268:162–168. doi: 10.1006/excr.2001.5287. [DOI] [PubMed] [Google Scholar]