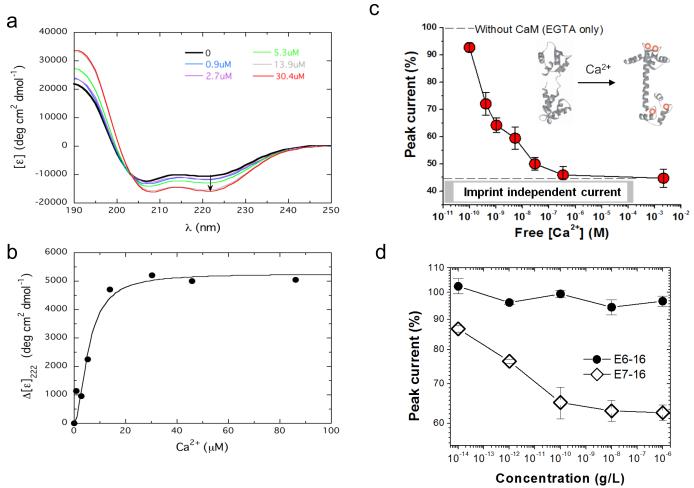
Figure 4.
Detection of calcium-dependent calmodulin conformational changes and HPV derived oncoproteins. a, Far-UV CD spectra showing the molar ellipticity (measured in deg-cm2 dmol−1) for calmodulin in the absence (black) and presence of 0.9 (blue), 2.7 (purple), 5.3 (green), 13.9 (grey), and 30.4 (red) μM Ca2+. The arrow shows increasing negative ellipticity at 222 nm. b, Change in calmodulin mean residue ellipticity at 222 nm ([ε]0 – [ε]Ca2+) as a function of added Ca2+. The increasing value for Δ[ε] indicates an increase in helix as Ca2+ is added. c, Nanosensor DPV represented as the percentage of peak current versus free Ca2+ concentration. The peak current is normalized to that recorded in Tris buffer with 1 mM EGTA (see Supplementary Information for details). Imprint-independent current represents the total residual current with overloaded Ca2+. d, MI recognition of oncoprotein E7 type-16 by an E7-16 imprinted sensor. Protein E6-16 was used as a negative control. Data represent percentage of peak current vs. E7 (open diamonds) or E6 (closed circles) type 16 protein concentrations. Error bars show standard error of the mean (n=3).