Table 1.
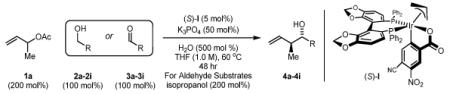
Enhanced levels of anti-diastereo- and enantioselectivity in alcohol mediated carbonyl crotylations using the chromatographically isolated single component iridium catalyst (S)-I.a
|
In Situ Method (ref. 2c) [Ir(cod)Cl]2 (2.5 mol%) (S)-SEGPHOS (5 mol%) 4-CN-3-NO2BzOH (10 mol%) Cs2CO3 (20 mol%) THF (2.0 M), 90 °C, 48 hrs α-Methyl Allyl Acetate (200 mol%) For Aldehyde Substrates isopropanol (200 mol%) |
 |
||
|---|---|---|---|
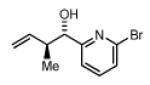
| 2a,3a: R = p-Br-Ph 2d,3d: R = 6-Br-2-Pyr 2g,3g: R = (CH2)2Ph |
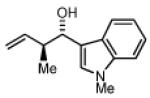
2b,3b: R = p-MeO-Ph 2e,3e: R = 3-Indolyl 2h,3h: R = (CH2)2NHBoc |
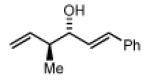
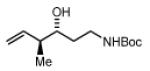
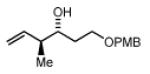
2c,3c: R = p-(CO2Me)-Ph 2f,3f: R = HC=CHPh 2i,3i: R = (CH2)2OPMB |
|
| Oxidation Level |
|
|
|
| Alcohol Aldehyde |
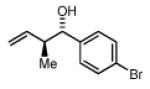
Preformed (S)-I 78% Yield 4a, 16:1 dr, 97% ee 82% Yield 4a, 17:1 dr, 98% ee |
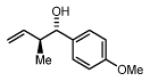
Preformed (S)-I 91% Yield 4b, 10:1 dr, 95% ee 89% Yield 4b, 12:1 dr, 98% ee) |
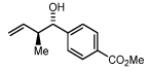
Preformed (S)-I 78% Yield 4c, 11:1 dr, 98% ee 81% Yield 4c, 13:1 dr, 98% ee |
| Alcohol Aldehyde |
In Situ (S)-I 73% Yield 4a, 8:1 dr, 95% ee 78% Yield 4a, 11:1 dr, 97% ee |
In Situ (S)-I 67% Yield 4b, 5:1 dr, 90% ee 75% Yield 4b, 7:1 dr, 97% ee |
In Situ (S)-I 70% Yield 4c, 7:1 dr, 95% ee 80% Yield 4c, 11:1 dr, 96% ee |
|
|
|
|
| Alcohol Aldehyde |
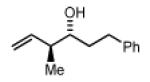
Preformed (S)-I 50% Yield 4d, 14:1 dr, 98% ee 75% Yield 4d, >20:1 dr, 97% ee |
Preformed (S)-I 75% Yield 4e, 7:1 dr, 98% ee 74% Yield 4e, 10:1 dr, 98% ee |
Preformed (S)-Ib 72% Yield 4f, 10:1 dr, 93% ee 77% Yield 4f, 10:1 dr, 98% ee |
| Alcohol Aldehyde |
In Situ (S)-I no product observed |
In Situ (S)-I 73% Yield 4e, 5:1 dr, 95% ee 78% Yield 4e, 6:1 dr, 97% ee |
In Situ (S)-I 61% Yield 4f, 7:1 dr, 86% ee 66% Yield 4f, 8:1 dr, 98% ee |
|
|
|
|
| Alcohol Aldehyde |
Preformed (S)-I 71% Yield 4g, >20:1 dr, 99% ee 71% Yield 4g, >20:1 dr, 98% ee |
Preformed (S)-Ib 71% Yield 4h, >20:1 dr, 96% ee 66% Yield 4h, >20:1 dr, 99% ee |
Preformed (S)-I 76% Yield 4i, 15:1 dr, 97% ee 76% Yield 4i, >20:1 dr, 99% ee |
| Alcohol Aldehyde |
In Situ (S)-I 69% Yield 4g, 7:1 dr, 98% ee 71% Yield 4g, 11:1 dr, 98% ee |
In Situ (S)-I no product observed |
In Situ (S)-I 73% Yield 4i, 7:1 dr, 95% ee 88% Yield 4i, 7:1dr, 95% ee |
Cited yields are of material isolated by silica gel chromatography. Enantiomeric excess was determined by chiral stationary phase HPLC analysis through comparison of reaction products to racemic diastereomeric mixtures. For assignment of relative and absolute stereochemistry, see reference 2c. See experimental section for further details.
70 °C.
