Table 1.
Optimization of the hydroboration/allylboration
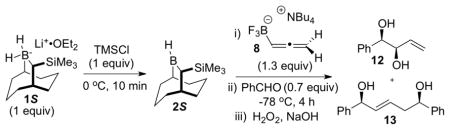 | ||||
|---|---|---|---|---|
| entry | solvent | hydroboration temp/time | % yield of 12a (syn/anti)b | ratio 12:13c |
| 1 | CH2Cl2 | 0 °C/1 h | 39 (5.7:1) | 3.0:1 |
| 2 | CH2Cl2 | −10 °C/1 h | 38 (16:1) | 2.9:1 |
| 3d | CH2Cl2 | −10 °C/1 h | 39 (12:1) | 3.3:1 |
| 4 | CH2Cl2 | −10 °C/3 h | 41 (5.1:1) | 3.2:1 |
| 5 | CH2Cl2 | −30 °C/1 h | 52 (> 20:1) | 3.7:1 |
| 6 | CH2Cl2 | −30 °C/3 h | 52 (> 20:1) | 4.3:1 |
| 7 | Et2O/CH2Cl2e | −30 °C/1 h | 50 (> 20:1) | 7.1:1 |
| 8 | toluene/THFf | −30 °C/1 h | 51 (> 20:1) | > 30:1 |
| 9 | toluene/CH2Cl2g | −30 °C/1 h | 87h (> 20:1) | > 30:1 |
Isolated yields.
Determined by 1H NMR analysis.
Determined after isolation of 12 and 13.
Dibal-H (2 equiv) added before the oxidation step.
Et2O/CH2Cl2 (2:1).
Toluene/THF (2:1).
Toluene/CH2Cl2 (15:1).
12 obtained in 97% ee, determined by Mosher ester analysis.
