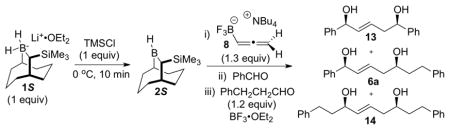
Table 2.
Optimization of the double allylboration leading to 6a
 | |||||
|---|---|---|---|---|---|
| entry | PhCHO (equiv) | BF3·OEt2 (equiv) | % yield 6ab | ratio E/Zc | ratio 13/6a/14c |
| 1 | 0.75 | 1.5 | 60 | > 20:1 | 0:89:11 |
| 2 | 0.85 | 1.5 | 73 | > 20:1 | 0:100d:0 |
| 3 | 0.95 | 1.5 | 51 | > 20:1 | 8:92:0 |
| 4e | 0.85 | 1.5 | 30 | 6:1 | 0:100:0 |
| 5f | 0.85 | - | 77 | 6:1 | 0:100:0 |
Reaction conditions: solvent: toluene/CH2Cl2; hydroboration: −30 °C, 1 h; first allylboration: −78 °C, 4 h; second allylboration: −78 °C, 4 h; workup: pH 7 buffer (KH2PO4/NaOH).
Isolated yields.
Determined by 1H NMR analysis.
6a obtained with > 20:1 dr and 97% ee, determined by Mosher ester analysis.
Second allylboration: 0 °C, 2h.
Second allylboration: −78 to 20 °C, 12 h.
