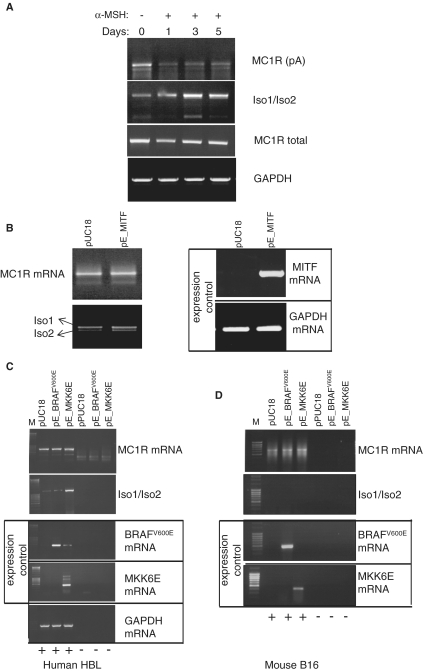
Figure 6.
Exposure of human melanocytes to α-MSH inversely affects expression levels of MC1R and MC1R-TUBB3 chimeric transcripts. (A) α-MSH treatment (10 nM) of HBL human melanoma cells results in a gradual increase in MC1R-TUBB3 chimera and reduced MC1R mRNA levels. RT–PCRs targeting all the above described transcripts originating from the MC1R locus were performed using total RNA isolated from HBL cells exposed to 10 nM α-MSH for 0, 1, 3 and 5 days, respectively (lanes 1–4) as indicated above the gel. The MC1R mRNA specific PCR is labelled: MC1R (pA) using PCR primers F1-dT, MC1R-TUBB3 chimera transcripts are denoted by (Iso1/Iso2) using PCR primers F1-R4, total transcripts from the MC1R locus (MC1R + MC1R-TUBB3) are labelled as (MC1R total) using PCR primers located in the 5′-UTR and the 5′-end of the MC1R ORF and GAPDH represents an additional control. (B) RT–PCR analysis of total RNA isolated from HBL cells transfected with a control pUC18 plasmid and a construct over expressing MITF (pE_MITF). Left panel showing results using primers amplifying MC1R mRNA and MC1R-TUBB3 chimera (Iso1/Iso2). Right panel, control RT–PCRs confirming over expression of MITF mRNA and GAPDH mRNA levels. (C) RT–PCR analysis of transfected HBL cells over expressing a constitutively active BRAF kinase (pE_BRAFV600E) or over expressing the constitutively active p38 specific MKK6 kinase (pE_MKK6E). RT–PCR results for MC1R specific primers (MC1R) (first panel) and chimera specific primers (Iso1/Iso2) (second panel) are shown. Control RT–PCRs confirming over expression of BRAF or MKK6 respectively are indicated by ‘expression control’ panel. A general control measuring unrelated GAPDH mRNA levels is presented (bottom panel). (+/−) indicates the presence or absence of reverse transcriptase in the RT reactions. (D) Identical analysis as shown in (C), but using mouse B16 melanocytes indicating that over expression of MKK6E does not result in the production of chimeric transcripts in mouse melanocytes.