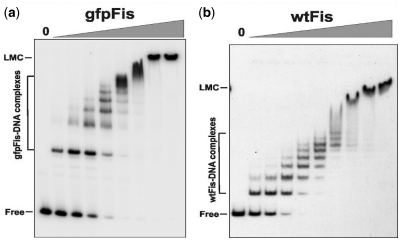
Figure 6.
Non-specific DNA binding by gfpFis and wtFis evaluated by gel mobility shift experiments. gfpFis (a) and wtFis (b) were incubated with a 150 bp 32P-labeled fragment from the S. cerevisiae MET14 gene and subjected to electrophoresis in a native 5% polyacrylamide gel. 0 designates no added protein followed by 2-fold increasing amounts of protein beginning at 1.3 nM for gfpFis and 1.1 nM for wtFis. Complexes containing from 1 to 6–8 dimers of Fis are formed with increasing amounts of added protein in both cases. The calculated Kd for the first bound complex was 3.1 ± 1.4 and 1.7 ± 0.5 nM and for the fully-coated DNA complex [seven Fis dimers, see ref. (14)] was 27.5 ± 7.8 and 32.0 ± 3.6 nM for gfpFis (n = 3) and wtFis (n = 5), respectively. At ≥70 nM both proteins also form a high-order complex referred to as the low-mobility complex (LMC). The slower relative migrations of the gfpFis complexes are consistent with the MW difference of the dimeric proteins: 76.6 kDa for gfpFis and 22.8 kDa for wtFis.