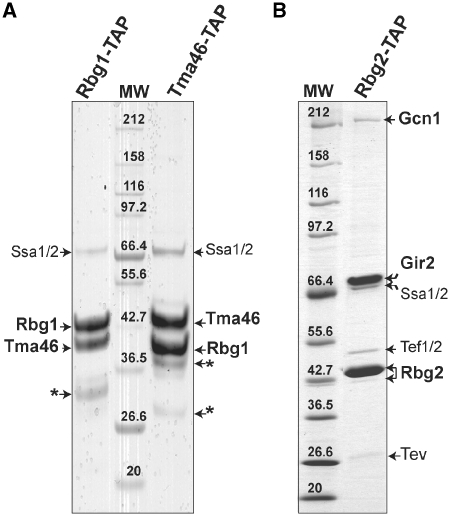
Figure 1.
Rbg1 and Rbg2 are not associated with the same protein partners in vivo. Protein profiles observed after TAP purification of the Rbg1-TAP or Tma46-TAP fusions (A) or Rbg2-TAP fusion (B). TAP purified proteins were fractionated on a 5–20% gradient SDS-PAGE gel and stained with Coomassie blue. Molecular weight markers are indicated (MW). Proteins identified by mass-spectrometry are indicated. Gir2 is a highly acidic 31-kDa protein and has an anomalous electrophoretic behavior (38). The protein chaperones, Ssa1 and Ssa2 also observed in other TAP purified purifications, are likely to be nonspecific interactants. Some remaining TEV protease was detected in the gel shown in (B). An asterisk indicates bands identified as degradation products of Rbg1 or Tma46 proteins.