Image-guided focused ultrasound surgery (FUS) effectively combines two technologies, MR imaging or US and FUS, into an image-guided therapy delivery system for noninvasive tumor ablation or the targeted delivery of drugs, both of which can either replace or complement surgery or radiation therapy.
Abstract
Focused ultrasound surgery (FUS) is a noninvasive image-guided therapy and an alternative to surgical interventions. It presents an opportunity to revolutionize cancer therapy and to affect or change drug delivery of therapeutic agents in new focally targeted ways. In this article the background, principles, technical devices, and clinical cancer applications of image-guided FUS are reviewed.
© RSNA, 2011
Introduction
Ultrasound was first described by Loomis and Wood in the 1920s (1) as having a biologic effect on living tissues. These two individuals have been described by some as “the fathers of ultrasonics” (2). Therapeutic ultrasound integrated with imaging is now one of the most rapidly expanding techniques in image-guided therapy. It combines the expertise in imaging and various minimally and noninvasive procedures. Many traditional surgical procedures have been replaced by minimally invasive image-guided approaches.
Thermal ablation occurs when the targeted delivery of heat or cold causes a rapid change in temperature (>55°C for heat or <−20–50°C for cold) in the local tissue environment. Imaging can be used to localize and target the thermal effects to the tumor for treatment, control the energy deposition, and assess treatment response (3). Currently, ultrasonography (US) and magnetic resonance (MR) imaging are used to guide therapeutic ultrasound, and the latter can provide all of these features, including quantitative temperature measurements in vivo through the temperature sensitivity of the water proton resonant frequency (4). The ability to use MR techniques to perform real-time thermometry makes MR the most reliable and comprehensive modality available for real-time (noninvasive) temperature monitoring. New US techniques that may allow for detection of thermal changes, through the use of contrast agents, and US-triggered drug delivery are under investigation (5–7).
Historical Perspective
The ability of FUS to induce thermal or mechanical effects at focal locations in living tissue has long been recognized; the earliest exploration of the medical use dates to 1942, when Lynn et al (8) first tested it in the brain. Since then, many researchers have investigated ultrasound-induced bioeffects and tested the technique for a number of different applications. Notably, in the 1950s the Fry brothers developed a clinical FUS device for treating patients with hyperkinetic disorders such as Parkinson disease. They used a complex sonication system that used x-rays to determine the target location with respect to skull bones and to focus the ultrasound beam through a craniotomy into deep brain tissues for functional neurosurgery (9,10). In the 1980s Lizzi developed the first U.S. Food and Drug Administration (FDA)-approved FUS system, trade name Sonocare CST-100, to treat ocular disorders such as glaucoma, and many patients were successfully treated.
Current research involves device development and the identification and validation of novel clinical applications. Transducer technology and array designs are constantly changing and improving. These developments include faster delivery of focal sonications, smaller devices that can be inserted into body orifices, and designs that conform to body topography. Newer applications beyond ablation, such as disruption of the blood-brain barrier (BBB), optimization of drug delivery, and thrombolysis, are of particular interest.
The clinical applications of FUS, which have been tested or are in clinical practice today, are primarily for tumor treatment. Several commercial FUS devices are in clinical use (Table 1). Most devices use US for guidance and are approved for clinical use in many parts of the world; however, their use is restricted in the United States with limited FDA clearance. The Ablatherm (EDAP TMS, Vaulx-en-Velin, France) or Sonablate (Focus Surgery, Indianapolis, Ind) devices are both approved and have CE mark approval in Europe and trials are underway for both in the United States. The acceptance of FUS in clinical practice in the United States has been relatively slow. There are multiple issues to explain this, including availability of equipment, cost of MR imaging, and expertise. The poor clinical outcomes reported have been related to lack of overall thermal control. Other issues have been the inability to clearly visualize the target tumor in deep-seated tumors using US guidance and even more importantly the inability to monitor the FUS ablation with real-time thermometry (Table 2). Many of these limitations can be overcome by using MR for target definition, thermometry, and guidance, as shown in the development of MR-guided FUS in (11,12).
Table 1.
Therapeutic FUS Companies with Oncologic Applications
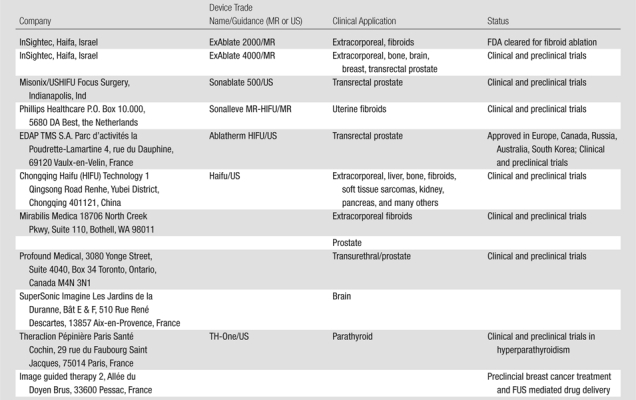
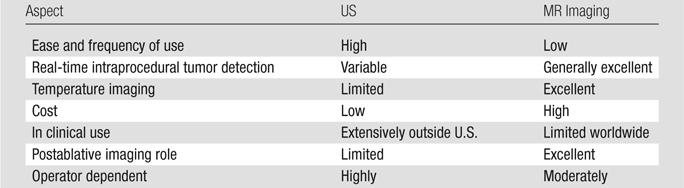
Table 2.
Comparison of US- and MR-guided FUS
In the early 1990s an MR-guided FUS device was developed in collaboration by researchers at Brigham and Women’s Hospital and General Electric. It used a single geometrically focused transducer adapted to perform in a high-field-strength MR magnet (12–14). Since the initial studies, substantial work has been done on developing MR imaging–compatible phased arrays and driving systems to increase the focal heating volume and steer the ultrasound beam and on MR imaging–based thermal dosimetry to guide the procedure. All components can be used within the MR suite with a custom designed table. Currently, one MR-guided FUS device, the ExAblate 2000 (InSightec, Haifa, Israel) has FDA clearance for the treatment of uterine fibroids. Work is underway on several other clinical targets for prostate, bone, and brain tumor treatments. Another MR-guided FUS system under development, the Sonalleve MR-HIFU (Philips Healthcare, Andover, Mass), is currently undergoing clinical trials for the ablation of uterine fibroids in Europe and the United States. This system has advanced MR-based control through integration of automated, real-time feedback control of the ultrasound energy deposition.
Basic Principles of Ultrasound and Transducer Design
Ultrasound propagates as mechanical vibrations that cause molecules within the medium to oscillate around their rest positions in the direction of wave propagation. The molecules forming compressions and rarefactions propagate the wave. As the ultrasound energy passes through tissue it is attenuated exponentially. The rate of energy flow through a unit area, normal to the direction of the wave propagation, is called the acoustic intensity. At 1 MHz the ultrasound wave is attenuated approximately 50% while it propagates through 7 cm of tissue. At 2 MHz the wave is reduced to approximately 25% of its initial value by the same tissue. The attenuated energy is mostly translated into temperature elevation in the tissue (15).
Ultrasound is effectively transmitted from one soft tissue layer to another with a small (few percent) amount of wave reflected back. At soft tissue–bone interfaces, approximately one-third of the incident energy is reflected back at normal incidence. In addition, the amplitude attenuation coefficient of ultrasound is about 10–20 times higher in bone than in soft tissues, a factor that causes the transmitted beam to be absorbed rapidly within the bone (16,17). Gas has a much lower density and sound speed than water or soft tissues and thus practically all of the energy is reflected back at the soft tissue–gas interface.
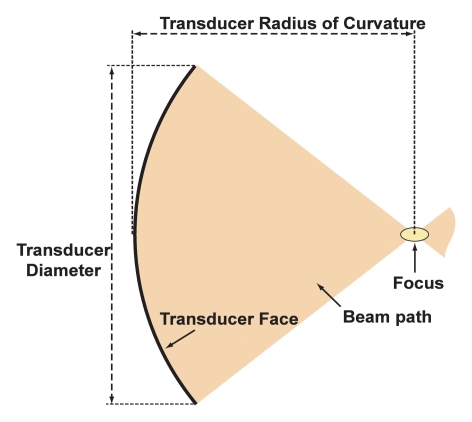
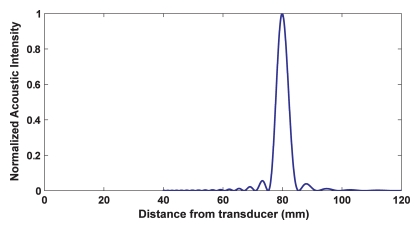
The ultrasound beams can be focused by using radiators, lenses, or reflectors. A focal diameter of 1 mm can be achieved in practice at 1.5 MHz with a sharply focused transducer. The length of the focus is typically 5–20 times larger than the diameter. Since the ultrasound beam is transmitted from an applicator 2–3 cm in diameter, the ultrasound intensity at the millimeter-sized focal spot can be several hundred times higher than in the overlying tissues. The ultrasound exposure drops off rapidly across the area within sonication path, thus, focusing provides a method to overcome attenuation losses and to concentrate energy deep in the body while sparing the overlying and surrounding tissues (Fig 1).
Figure 1a:
(a) Diagram shows properties of a geometrically focused transducer. (b) Normalized acoustic intensity along the direction of ultrasound propagation for a 1.5-MHz transducer with a radius of curvature of 8 cm and diameter of 10 cm.
Figure 1b:
(a) Diagram shows properties of a geometrically focused transducer. (b) Normalized acoustic intensity along the direction of ultrasound propagation for a 1.5-MHz transducer with a radius of curvature of 8 cm and diameter of 10 cm.
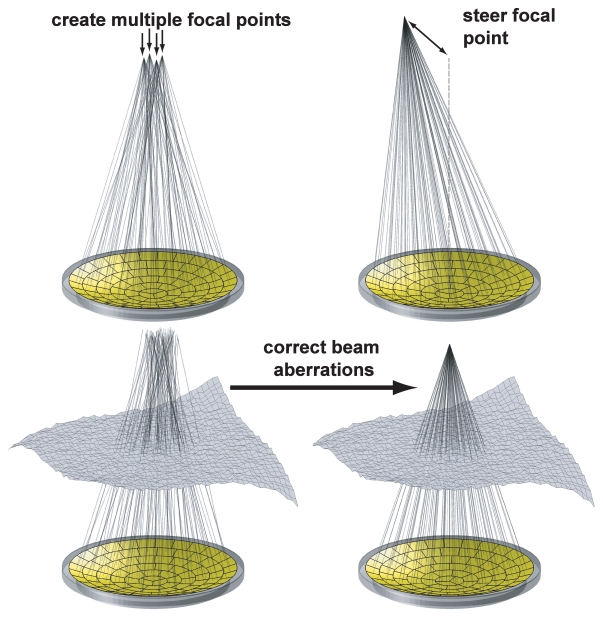
Improvements in focusing can be achieved by using transducer arrays that are driven with signals having the proper phase difference to obtain a common focal point (electrical focusing) (18). The main advantage of these phased arrays is that the focal spot can be directed electronically without the need to mechanically move the transducer (Fig 2). In addition, the electronic beam allows multiple focal points to be induced simultaneously or fast electronic scanning of the focus for example, to increase the size of the focal region (19–22). This latter feature allows for a decrease in the overall treatment time, which can be prohibitive in large tumors being treated with devices with a small focal zone.
Figure 2:
Ways a phased-array transducer can be used include producing multiple focal spots to increase the ablated volume per sonication, steering the focal point to different locations, and correcting for aberrations caused by tissue structures in the ultrasound beam path.
Bioeffects
The ultrasound energy absorbed by the body is converted to heat. The distribution of the temperature elevation during short exposures (a few seconds) closely follows the ultrasound field distribution. At longer exposures, thermal conduction and perfusion smooth the temperature distribution, resulting in less sharp temperature gradients. Due to the variations in the ultrasonic and heat transfer properties of the tissues, temperature elevation cannot be accurately predicted in vivo. Most notably, blood flow and perfusion are highly variable from location to location and from tissue to tissue. In addition, tissue close to large blood vessels will be cooled by the flow if the energy delivery is long or slow. Short/fast exposures (a few seconds) reduce the effect of blood perfusion on heat transfer and can coagulate tissues next to the large blood vessels, as has been shown by theoretical models and in vivo animal experiments (23–25). Therefore, short exposures can be used to reduce the variability in the thermal coagulation effects caused by blood flow and perfusion.
The degree of thermal damage to the tissue also depends on the temperature reached and the duration of the exposure. The effect of elevated temperature on cells and tissues has been found to be a nonlinear function of both time and temperature (26–29). For exposures of a few seconds, temperatures of approximately 60°C are adequate to cause irreversible tissue damage (30). The elevated temperature can also coagulate blood in small vessels and stops blood perfusion in the coagulated tissue volume (31). Lower temperature thermal exposures can be utilized for hyperthermia to sensitize tissue to radiation or chemotherapy, and to activate heat-sensitive genes (32) or drugs (33). When the temperature reaches 100°C, the tissue water boils, and gas bubbles are produced in the focal zone. These bubbles can reflect and scatter the ultrasound beam and significantly modify the energy deposition pattern. As described below, the bubbles can interact strongly with the ultrasound field, further complicating the situation. For these reasons, boiling is generally avoided during thermal ultrasound therapies.
At high pressure amplitudes, small gas pockets in fluids can grow into microbubbles by way of rectified diffusion. The formation and interaction of these microbubbles with the ultrasound field is referred to as acoustic cavitation. Preformed microbubbles, which are available clinically as US contrast agents, can also be used as cavitation sites with lower-intensity exposures (34). The ultrasonic pressure wave causes these bubbles to expand and contract, resulting in an increased energy loss from the beam. When the pressure wave amplitude is increased, the bubbles can collapse, causing shock waves with high pressures and shear forces that can cause direct mechanical damage to the tissue, a phenomenon known as inertial cavitation. Sonications resulting in inertial cavitation can induce mechanical tissue destruction, which may offer some advantages over using heat alone for tissue ablation (35–37). In some cases, inertial cavitation (probably combined with high temperatures) can achieve focal tissue vaporization (38–40).
Cavitation effects have been shown to result in several bioeffects that may have clinical utility. Histologic studies have shown that cavitation can disrupt the BBB (41), cause selective vascular damage (42), generate tissue necrosis (41), and occlude deep arteries (43). In addition, animal tumor studies have shown that FUS-induced cavitation can activate certain chemicals (44). The disruption of arteriosclerotic plaques and thrombi are also cavitation-mediated events (45,46). Cavitation effects can also influence cell membrane (47) and vascular (48) permeability. Given all that is understood about its mechanical effects, cavitation offers new therapeutic options.
In addition to heating and effects related to microbubbles oscillation and collapse, other ultrasound effects may have clinical applications. Radiation force, which occurs along the direction of the ultrasound beam, may be involved in the enhancement in increased drug delivery to tumors observed with pulsed sonications (49). Acoustic streaming in fluids (15) is thought to be involved in the clinical observation of enhanced effectiveness of tissue plasminogen activator in treating stroke when the vessel is exposed to a pulsed ultrasound beam at diagnostic pressure levels (50). Finally, extensive studies have shown that ultrasound accelerates bone and wound healing, though the basic mechanism for this is not completely understood (51,52).
Image Guidance Modalities: Overview and Comparison
US Guidance
By using US guidance, many pioneering FUS procedures have been performed (53). The results of combining US imaging for tumor identification and targeting with therapeutic ultrasound have been so compelling that large numbers of procedures in the liver, breast, and in the soft tissue for tumors, such as sarcomas, have been documented (54). The goals of treatment range from intent to cure/total tumor ablation to local tumor/pain reduction or palliation. The advantages of this combined approach are ease of use, relatively low cost, portability, and patient access (Table 2).
Any image guidance technique must accurately localize the target tumor, its boundaries, and surroundings for targeting the acoustic beam through acoustic windows and should be able to monitor the temperature elevations to ensure a safe therapeutic thermal dose. US imaging is variable, inconsistent, and operator dependent in the detection of exact tumor margins and may fail to depict the detailed locoregional anatomy. For example, in the imaging of tumors of the prostate, the visualization of the normal and abnormal prostate tissue and surrounding structures all are superior with MR imaging. This has been shown both in diagnostic studies and for FUS (55). US is an excellent imaging tool for more superficial soft tissues, such as breast. As the tissue characterization of US improves, using methods such as strain/elastography, it is likely to provide increased tissue characterization. However, MR of the breast has significantly improved tumor detection and depiction of margins compared with conventional US (56).
Furthermore, using US it can be difficult to reliably reproduce the same image/location over time. Bone and air cause artifacts that impede visualization. The location of the focal spot of FUS generally cannot be seen with US during therapy, and the temperature elevations cannot be measured by using standard US imaging methods.
As US oncologic imaging methods improve and US-based thermometry or tissue coagulation detection (57,58) or other means to guide FUS ablation become clinically available, US imaging will likely continue to play a major role in guiding clinical FUS (59).
MR Guidance
Combining methods of thermal ablation with advanced acoustic transducer technology using MR imaging guidance and MR imaging thermometry allows for accurate targeting, real-time monitoring, and even control of energy deposition. MR imaging can provide imaging of the target tumor, detection of its margins, visualization of the surrounding anatomy, and monitoring of temperature changes during therapy, thus providing a real-time closed loop system (11,13,60,61).
Some MR imaging parameters (such as T1-weighted, diffusion) are temperature sensitive and have been evaluated as methods for guiding thermal therapies (62,63). One parameter, the water proton resonant frequency, has been found to be particularly well suited for thermometry. The water proton resonant frequency changes with temperature through heat-induced changes in the hydrogen bonds in water, which affect electron screening of the nucleus (64). This can be mapped by using phase difference images generated from gradient echo sequences (65) (Fig 3). Importantly, its temperature sensitivity does not depend significantly on tissue type (66) and is not significantly affected by the thermal coagulation process (67). These unique features are most important to the success of MR thermometry. The main limitations of the technique are its motion sensitivity and its inaccuracy in fat tissues.
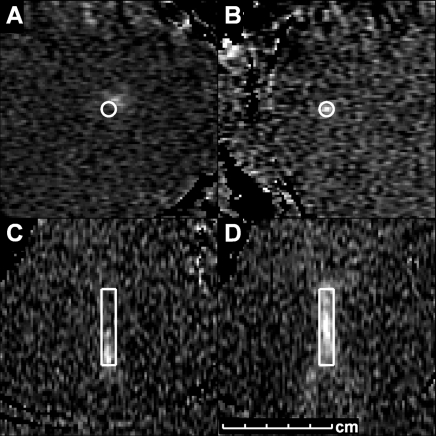
Figure 3:
Temperature images acquired during sonications to ensure correct targeting of the focal coordinate. A and C were acquired before the focal coordinate was corrected; B and D were acquired after correction. A, B, First, sonications were performed with temperature imaging perpendicular to the ultrasound beam direction. C, D, Next, sonications were performed with imaging along the beam direction. (Reprinted, with permission, from reference 149.)
The localization of tumor margins or the “treat/no treat boundary” is well visualized with MR, in general, more so than other guidance methods. Typically T2- or diffusion-weighted sequences are used during procedures (68). The inherent tissue contrast afforded by MR provides very high sensitivity to tissue abnormalities. The success of MR-guided FUS is thus related to the diagnostic imaging capabilities and thermal maps derived from MR data that result in high-quality target definition and tumor ablation.
The introduction of 3-T MR imaging in clinical practice is a major advance. The increased signal-to-noise ratio at this field strength allows for imaging at higher resolution and may improve tumor margin definition compared with imaging at 1.5 T.
Despite the ever-improving ability of MR imaging to depict cancerous tissue, it may not reveal the full extent of some malignant tumors because of ill-defined tumor margins, and its sensitivity can be limited in detecting the presence of a relatively small volume of tumor cells that may be present at the edges of a lesion and which infiltrate normal surrounding tissue. Newer and novel molecular imaging modalities, such as positron emission tomography (PET), advanced US techniques, optical, and advanced MR used with or without tumor-seeking contrast agents and biomarkers, all have potential to improve the detection of tumors, define their margins, and result in complete tumor ablation.
Clinical Applications
Applications for FUS as a cancer treatment have been diverse, ranging from ablation of focal breast cancer to palliative pain control from focal bone metastases. As the techniques improve capabilities such as transcranial transmission will lead to new applications and for brain tumors with improved ablation rates (69). Transcranial transmission (70,71) can also allow for opening of the BBB, allowing novel pharmacologic treatment of cancer of the brain. All offer new clinical applications to areas previously off limits.
Breast
Breast tumors are well suited to noninvasive treatment with FUS, as they are superficial, can be well defined by means of MR, and can be accessed with relative ease through the skin. Contrast material–enhanced diagnostic MR imaging, a modality that is more accurate than US or mammography, optimally determines the extent of breast cancer. In particular, using 3 T, MR can depict intraductal spread more accurately than US (72,73). MR also has a role for accurate localization in radiation therapy or in breast-conserving surgery to minimize local recurrence (74). MR may also lead to a reduction in high re-excision rates for lumpectomies and margin-positive partial mastectomies (75).
The treatment of breast fibroadenomas and focal breast cancer is both safe and feasible with FUS (76–78). Initial MR-guided FUS clinical trials in breast treatments were performed using a single-channel transducer, in benign fibroadenomas (76) and in breast cancer patients (Fig 4) who were not candidates for (or refused) surgery (79).
Figure 4:
Contrast-enhanced T1-weighted images (subtraction image) of a localized breast cancer mass (left) before and (right) after MR-guided FUS. The size of the region of nonperfusion (green outline) is larger than the original mass and includes a surgical margin. An adjacent area of contrast enhancement (arrow) may be difficult to distinguish from residual tumor, and thus would require further treatment before ending the procedure.
US imaging has also been used to guide FUS ablation of breast cancer. Wu et al (80) performed a randomized controlled trial of women with localized breast cancer, with the Haifu/US device (Chongqing Haifu Technology, Chongquing, China) using US guidance for tumor margin definition. In correlation with pathologic findings from mastectomy specimens, Wu et al (81) demonstrated typical coagulative necrosis, and the treatment margin with normal tissue was a rim of congestion representing inflammation. Further findings revealed that tumor vessels were severely damaged, and immunohistochemical staining showed that the treated cells lost proliferation, invasion, and metastatic potential.
At the same time a phase I trial of MR-guided FUS for breast cancer began (ExAblate 2000; InSightec), used in conjunction with a 1.5-T magnet. Histopathologic evaluation of treated lesions in this study demonstrated a limitation of the technique, as limitations with residual tumor foci were noted in the periphery of the ablation margin, due to underestimation of the tumor/treatment volume (79). More recent studies have shown the clinical value of MR-guided FUS in several different patient subgroups, such as older women with severe comorbidities precluding surgery or when the MR-guided FUS was combined with tamoxifen.
There are trials ongoing in Japan to evaluate FUS as an alternative therapy to lumpectomy. These have shown that good local control can be achieved with MR-guided FUS (82).
Active areas of research that may further improve breast treatments include the use of advanced systems that reduce treatment time, and MR temperature mapping in fatty tissue to improve control. Treatment times can be decreased by either increasing the volume ablated per sonication, interleaving the sonications to allow for multiple delivered at the same time or possibly enhancement with microbubbles. These areas, along with the use of 3 T for image guidance, are leading to renewed interest in this approach for treatment of breast cancer.
Liver
Image-guided interventions have become a mainstream treatment for focal liver tumors. FUS has also been applied noninvasively for either focal ablation or palliative reduction of liver tumors. Many patients have been treated worldwide, mainly in China and the United Kingdom, with US imaging guidance (83). Zhang et al (84) have recently reported their experience in treating 203 patients with hepatocellular carcinoma. The treatment of liver tumors with FUS presents unique challenges, namely, obstruction by the ribs and the respiratory motion of the liver (85). The ribs block the ultrasound beam passage, limiting the volume and parts of liver that can be accessed for treatment; motion leads to inaccurate thermometry and challenges obtaining accurate targeting. Civale et al (86) have designed a technique of segmenting the transducer’s acoustic profile to allow liver treatments around the ribs. In some clinical programs, the overlying ribs are resected to allow for a clear acoustic window over the liver. This has been reported by Zhu et al (87) in 16 patients with hepatocellular carcinoma. Because of the liver motion, treatments to date have been performed either with general anesthetic and controlled apnea or with careful training and the use of repeated breath-hold periods. Much more clinical experience has been gained by using US-guided FUS for applications such as palliative reduction of metastatic tumor burden in the liver (88). Both US- and MR-guided FUS have been used for focal hepatocellular carcinoma treatment (89–91). More recent advances with US-guided FUS have shown the advantage of combining it with transcatheter arterial chemoembolization (TACE). For example, Wu et al (92) showed that in a prospective study of 50 patients, an increase in median survival time occurred for patients with stage IVA hepatocellular carcinoma who underwent US-guided FUS. The patients in the FUS/TACE group survived a median time of 11.3 months versus 4.0 months for those in the TACE-only group.
Several clinical trials with MR-guided FUS have been performed in a small number of patients with liver tumors. These trials have been limited to tumors accessible below the ribs and have been performed with patients under general anesthesia, using controlled apnea (Fig 5). To enable MR-guided FUS in freely breathing patients, groups have investigated the use of motion-compensated MR imaging sequences and guiding the ultrasound beam to follow the respiratory motion of the liver (93). In time, new transducer design and technologic advances in array shapes should lead to even more improved access to the liver during FUS. These advances along with motion correction techniques will likely accelerate the growth of the application of FUS for focal liver lesions. Percutaneous ablative techniques, such as radiofrequency ablation or cryotherapy, are now used widely for ablation of both primary hepatocellular carcinoma and liver metastases. These have advantages over FUS as they are now well established and clinically accepted. Large tumors up to 5 cm can be ablated in 2–3 hours (85). However, FUS is noninvasive and can be performed repeatedly, as needed. FUS can also be accurately directed to treat complex three-dimensionally shaped tumors. Ultimately, prospective clinical trials and the results of further technologic advances will determine the role of FUS as a therapeutic tool in the liver.
Figure 5:
MR-guided FUS of hepatocellular carcinoma. Series in sagittal and axial planes show a small focal area of treatment; green regions = location of current sonication, blue areas = those that have been treated to the desired temperature in left lobe of the liver. Bottom left: Final posttreatment image in coronal plane shows entire thermal dose history. Bottom right: Image after intravenous gadolinium administration shows the focal nonperfusion. (Image courtesy of Wadyslaw Gedroyc, MD, St Mary’s Hospital, London, England.)
Prostate
New focal therapies for prostate cancer are increasingly being sought out by and used in patients who are seeking cancer control with reduced side effects (94). FUS treatment of prostate cancer has been first reported using US-guided FUS (95). The majority of prostate cancer patients who have been treated with focused US have been treated with one of two transrectal devices: Ablatherm (EDAP TMS) or Sonablate (Focus Surgery) (95–98). These devices integrate an FUS transducer into a rectal probe and use US imaging to plan and monitor the treatment. Unfortunately, grayscale US is limited in its depiction of focal tumors, which can be either hypo- or hyperechoic. Furthermore, during sonications, US imaging does not accurately depict thermal changes within the heated zone. However, hyperechoic regions may appear, presumably due to cavitation through the boiling or the intense negative pressures associated with high-intensity ultrasound exposures. These regions, while not predictive of the resulting thermal lesions, provide some level of feedback to the operator. There is considerable effort underway to improve the technique. Research groups have investigated US-based thermometry methods (99–102) and US elastography methods to detect thermal coagulation (103).
With use of spinal or general anesthesia, FUS of prostatic lesions can be performed on an outpatient basis. The operator adjusts the power output according to evolving conditions within the prostate and the proximity of the focus to neighboring structures, including the neurovascular bundle and rectal wall.
Successful treatment is defined by radiologic, pathologic, and biochemical markers of disease. More than a decade ago, Madersbacher et al (104) presented details of successful prostate FUS treatments. Subsequent trials have reinforced this, showing 54%–77% of patients free of disease 5 years after treatment. Blana et al (96) identified more than 75% of patients disease free at 5 years; however, a significant proportion of these patients had received neoadjuvant hormonal therapy, which may have affected the results. Misrai et al (97) found that prior treatment with hormonal therapy had a positive effect on the outcome with FUS. This study showed that the long-term results of FUS were worse when no hormonal therapy was used. In their study, the high-risk population, without hormonal therapy, was most likely to develop biochemical failure following FUS.
Adverse effects from US-guided FUS, as published by Thuroff et al (95), include urinary retention (8.6% patients), stress incontinence (13.1%), bladder outlet obstruction (3.6%), urinary tract infection (13.8%) and urethrorectal fistula (1.2%). Other studies have identified greater degrees of impotence, in approximately 50% of patients. These rates of adverse effects reflect results from early trials. However, newer improved devices will likely lead to reduction in the reported side effects. US-guided FUS for prostate cancer can be a useful and sometimes successful treatment option. The best results will most likely be obtained in a well-defined patient population subgroup with either FUS alone or synergistic with adjuvant or neoadjuvant hormonal therapy.
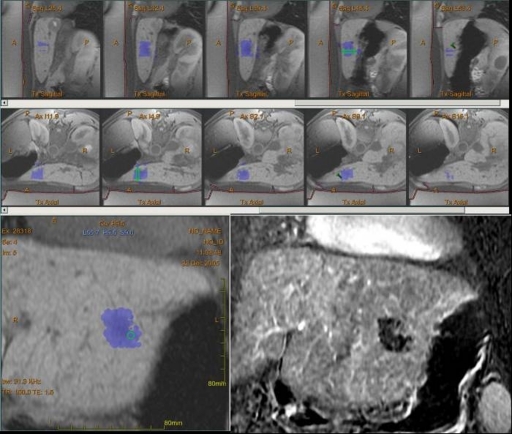
Newer approaches using MR as the imaging guidance method offer a potential advantage over US by allowing improved thermometry and tumor visualization. It is hoped that this will lead to reduced adverse effects, as it more accurately monitors temperature in the volume of ablation allowing for real-time adjustment of energy delivered. We and others are investigating MR-guided FUS using a transrectal approach in a canine model as a potential therapeutic approach for prostate cancer (22,105,106) (Fig 6). Others are investigating the use of transurethral ultrasound devices for MR-guided thermal ablation (107,108). The first human clinical trial is underway with MR-guided FUS performed prior to radical prostatectomy (109). Transducers have been positioned on a transurethral device or on an intrarectal device, to deliver the ultrasound beam. Clearly the engineering challenges involve the need to ensure safe and effective delivery of the ultrasound beam in the MR environment and with the anatomic constraints presented by the prostate and its locoregional structures. For safe and effective treatment the operator will need to have full control of the focal spot size and localization, to avoid heat delivery to critical structures such as the neurovascular bundles. We have demonstrated the use of 3-Slicer, an open-source software program, to track the positions of the neurovascular bundles in response to radiation therapy (110). A similar approach using online image-guided navigation during treatment could be considered.
Figure 6:
Preclinical MR-guided FUS of the prostate. Three images from an animal study show, A, axial phase image with thermal change (high-signal-intensity area centrally) from an individual sonication delivered by transrectal FUS transducer device (Insightec), then, B, axial T1-weighted and, C, sagittal T1-weighted images after the procedure and after injection of intravenous gadolinium show the focal ablation as an area of nonperfusion (yellow).
A prerequisite for focal FUS treatment in the prostate is better tumor definition with imaging (55,111). Current techniques with 3-T MR imaging are showing significant advances, especially when a combined multiparametric approach is used (112). Thus, the future of MR-guided FUS at 3 T offers a major advance for the field of FUS and, more importantly, for the men with prostate cancer seeking a noninvasive treatment option.
Bone
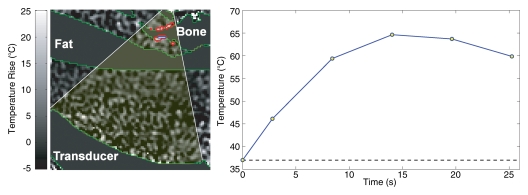
FUS has been used in clinical practice for treatment of primary bone tumors and palliation of pain from bone metastases. Initial experience for pain relief is very promising (113). It has great potential to be valuable in patients who cannot receive more radiation to a particular site, due to limitations in normal tissue tolerance or as the risk of fracture in the peri-irradiation period would be very high. The FUS treatment for palliation of pain from bone metastases differs from tumor ablation. Due to the high absorption in bone, the ultrasound energy requirements for FUS ablation in bone are much lower than in other targets; approximately only 30% of the soft tissue energy is required. One can take advantage of the high absorption in bone by placing the focal point beyond the bone surface. Because very little of the energy is transmitted into the bone, the ablation is localized to the periosteal bone–soft tissue interface (Fig 7). The pain palliation is thought to be due to FUS denervation of the nerves in the periosteal layer of bone.
Figure 7a:
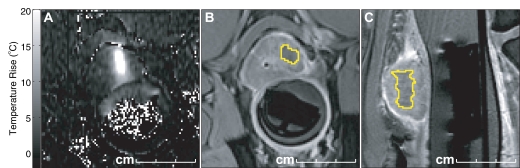
MR-guided FUS of bone tumors. (a) Schematic of bone treatment. FUS beam is oriented so its axis of propagation is normal to the bone surface. The focal point is placed behind the bone surface. Because absorption in bone is so much greater than in soft tissue, a large area can be heated in a single sonication. (b) Axial T2-weighted image in patient undergoing MR-guided FUS for large bone metastasis in right acetabulum. The transducer is directly posterior to the pelvis on the right. (c) Axial-oblique echo-planar imaging temperature map during MR-guided FUS. The bone surface is heated, raising the temperature in the adjacent tumor above the ablation area. Temperature drops off rapidly away from the bone. Red contours = regions that reached a thermal dose of at least 240 equivalent min at 43°C. The bone in the beam path for this sonication was fairly thin, and both sides of the bone were heated.
Figure 7b:
MR-guided FUS of bone tumors. (a) Schematic of bone treatment. FUS beam is oriented so its axis of propagation is normal to the bone surface. The focal point is placed behind the bone surface. Because absorption in bone is so much greater than in soft tissue, a large area can be heated in a single sonication. (b) Axial T2-weighted image in patient undergoing MR-guided FUS for large bone metastasis in right acetabulum. The transducer is directly posterior to the pelvis on the right. (c) Axial-oblique echo-planar imaging temperature map during MR-guided FUS. The bone surface is heated, raising the temperature in the adjacent tumor above the ablation area. Temperature drops off rapidly away from the bone. Red contours = regions that reached a thermal dose of at least 240 equivalent min at 43°C. The bone in the beam path for this sonication was fairly thin, and both sides of the bone were heated.
Figure 7c:
MR-guided FUS of bone tumors. (a) Schematic of bone treatment. FUS beam is oriented so its axis of propagation is normal to the bone surface. The focal point is placed behind the bone surface. Because absorption in bone is so much greater than in soft tissue, a large area can be heated in a single sonication. (b) Axial T2-weighted image in patient undergoing MR-guided FUS for large bone metastasis in right acetabulum. The transducer is directly posterior to the pelvis on the right. (c) Axial-oblique echo-planar imaging temperature map during MR-guided FUS. The bone surface is heated, raising the temperature in the adjacent tumor above the ablation area. Temperature drops off rapidly away from the bone. Red contours = regions that reached a thermal dose of at least 240 equivalent min at 43°C. The bone in the beam path for this sonication was fairly thin, and both sides of the bone were heated.
A recent multicenter trial presented by Lieberman et al (113), using the ExAblate device, in 31 patients showed a significant reduction of the pain, as recorded on the visual analogy scale, in 72% of patients at 3 months after treatment. They also reported a 67% reduction in the use of opiod pain medication.
MR-guided FUS of Uterine Leiomyomas: A “Tumor” Model
Uterine fibroids are the most common benign neoplasm in women. These can be very symptomatic in some women and only mildly so in others. Treatment options for women with symptomatic fibroids range from the most aggressive approach, hysterectomy, to the least invasive—that is, to do nothing. Unfortunately many women end up living with their fibroid symptoms, despite severe anemia, chronic pain, or prolonged infertility. An expanding group of minimally or noninvasive treatment options are now available including myomectomy, and uterine artery embolization or FUS. FUS has been performed with both US and MR guidance (114,115). Recent studies show that fibroid symptom relief can also be obtained with US-guided FUS (114,116). MR-guided FUS, is performed as an outpatient procedure, taking several hours with short recovery and with minimal postprocedure disability. MR-guided FUS treatment for uterine fibroids can be performed with the ExAblate 2000 (InSightec) device in a 1.5-T MR magnet. The device is part of a custom-made table that docks in the imager like a normal MR table. MR images are used to define the target volume, which is then analyzed in all three planes with superimposed ultrasound beam paths so as to ensure the following important safety considerations: (a) No beam passes through or near any bowel loops, (b) no beam passes through the bladder or major scar tissue, and (c) no distal beam passes close (no less than 4 cm) to the sciatic nerve or branches in front of the sacrum. The procedure begins with the delivery of low-power (20–50 W) sonication, with real-time thermometry performed simultaneously. The resultant images provide feedback on location, allowing the operator to determine the correct placement of the focal spot. The temperature images show the location of the low-power spot and, if correct, allow for the procedure to continue with increases in power applied gradually, up to a therapeutic dose usually over 70°C (117).
Once the therapeutic thermal dose at each location is achieved, the procedure continues with the delivery of all planned sonications. At the end of the procedure, imaging with contrast enhancement is performed to demonstrate the nonperfused necrotic tissue in the fibroid (Fig 8).
Figure 8a:
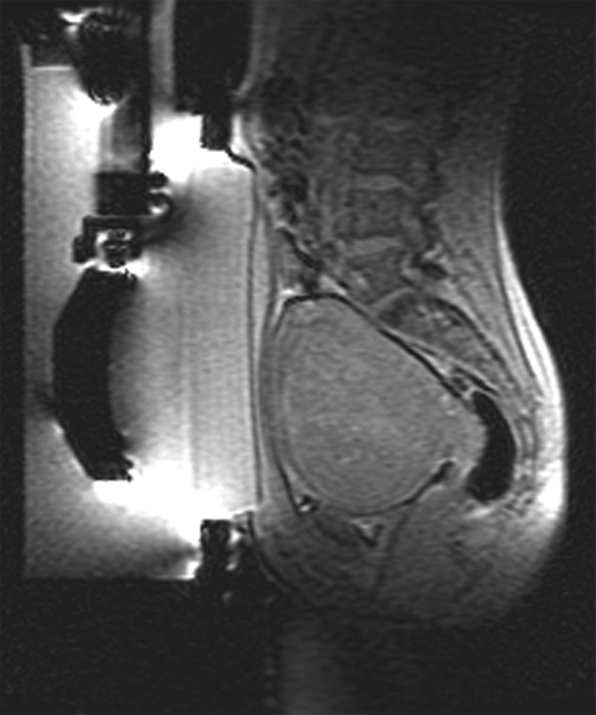
MR-guided FUS of uterine fibroid. (a) Sagittal image shows patient set up and the FUS transducer system on the left. Images (b) before and (c) after intravenous gadolinium administration, acquired immediately after treatment, demonstrate the focal nonperfused fibroid as a result of the ablation.
Figure 8b:
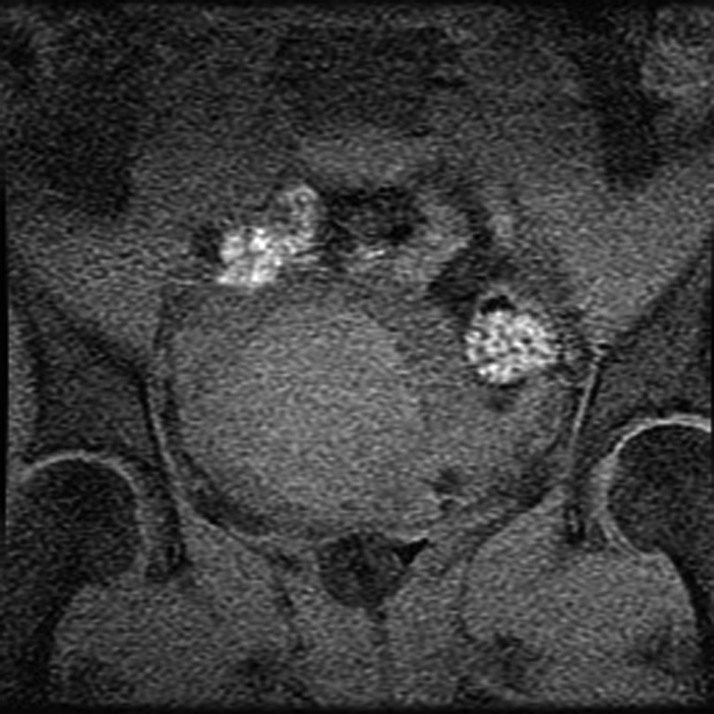
MR-guided FUS of uterine fibroid. (a) Sagittal image shows patient set up and the FUS transducer system on the left. Images (b) before and (c) after intravenous gadolinium administration, acquired immediately after treatment, demonstrate the focal nonperfused fibroid as a result of the ablation.
Figure 8c:
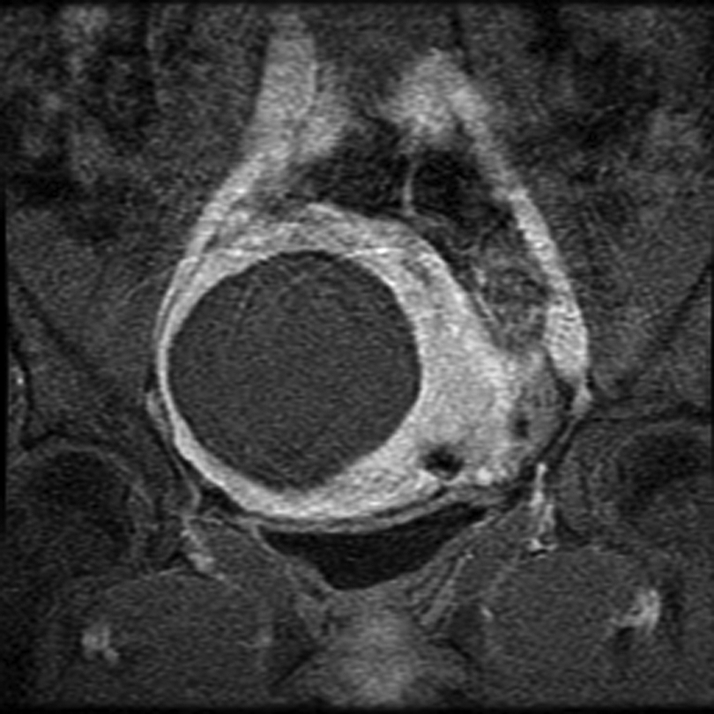
MR-guided FUS of uterine fibroid. (a) Sagittal image shows patient set up and the FUS transducer system on the left. Images (b) before and (c) after intravenous gadolinium administration, acquired immediately after treatment, demonstrate the focal nonperfused fibroid as a result of the ablation.
Several reports have detailed the results of multicenter clinical trials of MR-guided FUS in the treatment of leiomyomas, dating from the first report in 2003 (115) to later ones from 2005 and 2006 (118–120). Another important lesson from the clinical trials is that the greater the treatment volume, the better the response achieved. After the safety of MR-guided FUS treatment of fibroids was demonstrated, the FDA broadened the treatment guideline in 2004 to allow a treatment volume of 150 mL per fibroid with no restriction on the distance of the lesion from the endometrium. Fennessy et al (121) first analyzed the effect of the different protocols on treatment results. The protocols differed in treatment volume, which were changed to allow larger treatment volumes after small volume treatments were demonstrated to be safe. The authors found that the relaxed treatment protocol resulted in a greater nonperfused volume, with 26% achieved versus the 17% of the restricted protocol (P < .001). For those treated with the restricted protocol, results of clinical follow-up showed that 74% of patients at 6 months and 73% at 12 months had a greater than 10-point improvement in symptom score. In those treated, according to the relaxed protocol, 88% reported a 10-point or greater symptom improvement at 6 months, and 91% had significant symptom improvement at 12 months. The results proved that the less restrictive treatment protocol resulted in a larger treatment volume and better symptom improvement and durability (122).
MR-guided FUS has been broadly accepted as an option for the treatment of uterine fibroids. To date, more than 6000 patients have been treated with MR-guided FUS worldwide, with more than 5400 performed in routine clinical practice. In the recent reports of clinical experience, with proper selection of patient population, the treatment volume is often more than 50% of the total fibroid volume (121). The report of Mikami et al (90) showed the mean percent nonperfused volume ratio in the 6-month follow-up was 47%. Similarly, in the report by Morita et al (123), the mean percent nonperfused volume was 60% immediately on completion of MR-guided FUS treatment, with the fibroids shrinking by about 39% at 6 months.
In summary, based on clinical outcomes that have been reported, MR-guided FUS outpatient-based treatment is technically feasible and reproducible and significantly reduces symptoms in more than 75% of women with fibroids treated. The best results are seen when the nonperfused volume is high and recommendations are to achieve at least 60% nonperfused volume.
The rate of side effects is generally low. Transient adverse effects include mild skin burn, nausea, short-term buttock or leg pain, and transient sciatic nerve palsy (121,122). In one case, severe skin burn has been reported (124). Another ongoing evaluation of the clinical application of MR-guided FUS of uterine fibroids is its effect on fertility. A clinical trial is underway to evaluate the efficacy and safety of MR-guided FUS in the enhancement of fertility in women with nonhysteroscopically resectable uterine fibroids who have a diagnosis of otherwise unexplained infertility (125,126).
Technical Improvement
One of the main limitations of FUS in treating large tumors such as uterine fibroids is the long treatment time required. Initially the approach to clinical treatment has been conservative to avoid the thermal build-up that occurs along the ultrasound beam path when multiple overlapping sonications are delivered in succession (127). To avoid this build-up, long delays were required between sonications to allow cooling of the treated tissue. However, since the initial trials, newer strategies have been developed, including the use of the so-called interleave mode, to increase the treatment volume and decrease treatment time. By redefining the sonication order by minimizing overlap between sonications, less heat absorption in the tissues through which the ultrasound beam passes—known as the “pass zone”—can be achieved, and cooling time can be largely reduced. Others have investigated using this thermal build-up to their advantage by using long sonications that steer the focal point in a pattern (128,129).
Another promising advance is “enhanced sonication,” which uses microbubbles to enhance the heating at the focal point without reducing the overall energy deposition rate (105,130,131). This technique uses cavitation—a nonthermal mechanism to cause physical destruction of tissue and cells. In contrast to a continuous power transmission that lasts about 20 seconds to gradually heat at the focus, the enhanced sonications use high-power bursts to generate microbubbles that cause energy reflection and greater energy absorption and heating at the spot location. Therefore, although the total energy is the same, the volume of tissue ablated per individual sonication/treatment spot can be enlarged to allow for larger treatment volumes in shorter time periods. To determine the reliability and efficacy of enhanced sonication, clinical tests have recently begun (132).
Brain
Traditional neurosurgical approaches to deep-seated tumors usually result in some degree of normal brain damage due to the dissection down to the tumor. It has been known since the mid-20th century that FUS can destroy targeted tissue without injuring the normal brain and thus there has been extensive research to develop FUS as an “ideal” neurosurgical method (10,133).
Most of that research involved open craniotomies (134) in which the ultrasound beam propagated directly into the brain. Nevertheless, it is known that the ultrasound beam can be directed transcranially to make the entire procedure noninvasive (70,71,135,136). In this procedure, ultrasound exposure is accomplished by using phased-array transducers surrounding the skull. The phase shifts caused by the irregular skull bone can be compensated for by the thickness measurements generated from x-ray computed tomography (CT) data. The phase corrections permit focusing at relatively lower frequencies. A prototype FUS phased-array research system for trans-skull brain tissue ablation using a 500-element US phased array operating at frequencies of 700–800 kHz has been developed (71). After designing a working transducer model, Hynynen and Jolesz (70) noted that a focus distorted by the insertion of a human skull fragment in a water bath could be restored by simply adjusting the driving phase of each element in a spherically curved transducer array with transducer elements large enough to make practical, high-gain arrays feasible. Initial work with phase conjugation used a small receiver placed in the head to demonstrate that it was possible to deliver a signal through the closed skull.
A second high-intensity focused ultrasound (HIFU) treatment system produced by SuperSonic Imagine (Aix en Provence, France), has been developed, with promising results in an animal model, showing focal brain thermal ablation without any skull heating. A clinical system designed for the MR-guided FUS thermal surgery of the brain through the intact skull has been developed and tested in rhesus monkeys (137) and in a small number of patients (106,138) (Fig 9). The ultrasound beam was generated originally by a 512-channel phased-array system (ExAblate 3000; InSightec).
Figure 9:
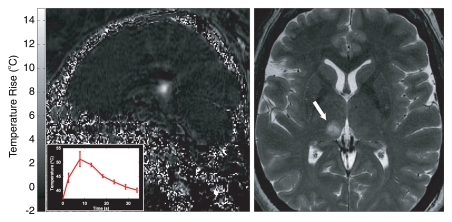
MR-guided FUS in the brain for functional neurosurgery. A right centrolateral thalamotomy was performed with transcranial MR-guided FUS in a 50-year-old female patient with chronic therapy-resistant neuropathic pain in the left trigeminal nerves V2 and V3. Two locations were sonicated to create a lesion of suitable size to cover the target area. Left: MR temperature map at peak heating (inset = temperature/time curve in 3 × 3 voxel region at the focal point). Right: Axial T2-weighted image shows the resulting lesion (arrow). (Images courtesy of University Children’s Hospital, Zurich, Switzerland.)
FUS can be used to treat various central nervous system diseases. These include lesions associated with epilepsy, pain, or Parkinson disease (10). It can also be used to selectively open the BBB to target drug delivery through the noncoagulative effects of preformed bubbles circulating within the vasculature. In fact, throughout the entire body, cavitation-related effects can facilitate deliver of drugs and gene therapy to targets.
The use of FUS to temporarily disrupt the BBB to target the delivery of drugs is a very exciting application (139,140). The BBB is the major challenge to delivering a variety of therapeutic agents in the brain. It is a composite of endothelial structures that are effective in excluding delivery over 98% of small-molecule drugs and almost 100% of large-molecule neurotherapeutics to the brain tissue. The BBB places enormous restrictions on the drugs which can be used in the central nervous system and constrains drug development. Conventional approaches for targeted drug delivery to the brain include direct injection or infusion into the brain substance using catheters and implantable devices (141). Recent approaches for drug delivery include the design of new drugs (or carriers) and use of the endogenous BBB carrier systems to shuttle drug molecules through BBB (142). While this can be a very effective approach, the amount of drug delivered may be small due to a limited number of receptors and a discrete quantity of molecules attached to the carriers. The drugs are also delivered everywhere in the brain, which may not be necessary or desirable.
To disrupt the BBB, ultrasound is used at very low power, with short FUS bursts combined with an injection of a US contrast agent. These contrast agents consist of preformed gas bubbles (diameter ∼ 1–5 μm) that circulate in the vasculature for a few minutes after a bolus injection. They serve to concentrate the effects of the beam to the blood vessel walls to induce BBB disruption. Without the agent, the low-power ultrasound bursts do not appear to produce any effects. The disruption is likely the result of mechanical stimulation and/or forces on the endothelial cells and the “tight junctions” that bind them together, resulting from bubble oscillation, acoustic streaming of the fluid surrounding the bubbles, and effects related to radiation force. Multiple effects may be responsible as both active and passive transport across the barrier have been observed (143).
This unique capability of noninvasively disrupting the BBB with FUS can allow even large molecular size agents such as antibodies to reach the brain (144,145). The disruption lasts for approximately 4 hours and can be achieved without any apparent damage to the brain (137,146). This has been demonstrated in animal studies and may be clinically feasible. If proved successful in patients, it would potentially represent a major advance in neuroscience in general and in oncology, as many advanced antibody-based chemotherapeutic agents cannot currently be used effectively in the central nervous system because of their large size and consequent blockage by the BBB. This approach can also be used with existing drugs that cannot pass the BBB, avoiding the expense and time needed to develop new drugs or to develop drug carriers for old drugs. It may be especially useful for the delivery of chemotherapy agents for brain metastases when effective agents are already available for treating the primary tumor and extracranial metastases.
Disruptive Technology
A completely noninvasive, image-guided, and controlled new therapy delivery system like FUS can revolutionize the various fields of surgery, radiation oncology, and other medical fields. This technology may be expensive, but the high cost is counterbalanced by reduced complication rates and hospitalization costs and, even more important, better outcomes (147). FUS may provide even more astonishing discoveries in the future (148).
In conclusion, image-guided FUS effectively combines two technologies, MR imaging or US and FUS, into an image-guided therapy delivery system for noninvasive tumor ablation or the targeted delivery of drugs, both of which can either replace or complement surgery or radiation therapy.
Essentials.
• This article reviews the basic principles of focused ultrasound surgery.
• The authors provide a comparison of guidance modalities.
• This article summarizes the worldwide clinical experiences in breast, liver, prostate, uterine, and brain tumors.
Disclosures of Potential Conflicts of Interest: C.M.C.T. Financial activities related to the present article: consultant to Insightec. Financial activities not related to the present article: travel/accommodations/expenses from GEHC Ad board. Other relationships: none to disclose. N.J.M. Author stated no financial relationship to disclose. K.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: stock/stock options in FUS Instruments. Other relationships: none to disclose. F.A.J. Financial activities related to the present article: consultant to Insightec. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose.
Received February 22, 2010; revision requested April 28; revision received September 13; accepted October 1; final version accepted December 21.
ExAblate clinical trials supported by Insightec, Haifa, Israel.
Funding: This research was supported by the National Institutes of Health (grants P41 RR019703 and R01 CA 109246).
Abbreviations:
- BBB
- blood-brain barrier
- FDA
- Food and Drug Administration
- FUS
- focused ultrasound surgery
- HIFU
- high-intensity focused ultrasound
References
- 1.Wood RW, Loomis AL. The physical and biological effects of high frequency sound waves of great intensity. Philos Mag Ser 7 1927;4(22):417–436 [Google Scholar]
- 2.Seabrook W. Doctor Wood, modern wizard of the laboratory: the story of an American small boy who became the most daring and original experimental physicist of our day—but never grew up. New York, NY: Harcourt, Brace, 1941 [Google Scholar]
- 3.Moonen CT. Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound. Clin Cancer Res 2007;13(12):3482–3489 [DOI] [PubMed] [Google Scholar]
- 4.McDannold NJ, Jolesz FA. Magnetic resonance image-guided thermal ablations. Top Magn Reson Imaging 2000;11(3):191–202 [DOI] [PubMed] [Google Scholar]
- 5.Lindner JR, Jr, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 2001;104(17):2107–2112 [DOI] [PubMed] [Google Scholar]
- 6.Klibanov AL. Preparation of targeted microbubbles: ultrasound contrast agents for molecular imaging. Med Biol Eng Comput 2009;47(8):875–882 [DOI] [PubMed] [Google Scholar]
- 7.Böhmer MR, Klibanov AL, Tiemann K, Hall CS, Gruell H, Steinbach OC. Ultrasound triggered image-guided drug delivery. Eur J Radiol 2009;70(2):242–253 [DOI] [PubMed] [Google Scholar]
- 8.Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol 1942;26(2):179–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers R, Fry WJ, Fry FJ, Dreyer LL, Schultz DF, Noyes RF. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J Neurosurg 1959;16(1):32–54 [DOI] [PubMed] [Google Scholar]
- 10.Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron 1960;ME-7:166–181 [DOI] [PubMed] [Google Scholar]
- 11.Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA. MR-guided focused ultrasound surgery. J Comput Assist Tomogr 1992;16(6):956–965 [DOI] [PubMed] [Google Scholar]
- 12.Hynynen K, Damianou C, Darkazanli A, Unger E, Schenck JF. The feasibility of using MRI to monitor and guide noninvasive ultrasound surgery. Ultrasound Med Biol 1993;19(1):91–92 [DOI] [PubMed] [Google Scholar]
- 13.Cline HE, Hynynen K, Watkins RD, et al. Focused US system for MR imaging-guided tumor ablation. Radiology 1995;194(3):731–737 [DOI] [PubMed] [Google Scholar]
- 14.Cline HE, Schenck JF, Watkins RD, Hynynen K, Jolesz FA. Magnetic resonance-guided thermal surgery. Magn Reson Med 1993;30(1):98–106 [DOI] [PubMed] [Google Scholar]
- 15.Wells P. Biomedical ultrasound. Boston, Mass: Academic Press, 1977 [Google Scholar]
- 16.Lehmann JF, DeLateur BJ, Warren CG, Stonebridge JS. Heating produced by ultrasound in bone and soft tissue. Arch Phys Med Rehabil 1967;48(8):397–401 [PubMed] [Google Scholar]
- 17.Hynynen K, DeYoung D. Temperature elevation at muscle-bone interface during scanned, focused ultrasound hyperthermia. Int J Hyperthermia 1988;4(3):267–279 [DOI] [PubMed] [Google Scholar]
- 18.Ebbini ES, Cain CA. Multiple-focus ultrasound phased-array pattern synthesis: optimal driving-signal distributions for hyperthermia. IEEE Trans Ultrason Ferroelectr Freq Control 1989;36(5):540–548 [DOI] [PubMed] [Google Scholar]
- 19.Fan X, Hynynen K. A study of various parameters of spherically curved phased arrays for noninvasive ultrasound surgery. Phys Med Biol 1996;41(4):591–608 [DOI] [PubMed] [Google Scholar]
- 20.Wan H, VanBaren P, Ebbini ES, Cain CA. Ultrasound surgery: comparison of strategies using phased array systems. IEEE Trans Ultrason Ferroelectr Freq Control 1996;43(6):1085–1098 [Google Scholar]
- 21.Fjield T, Hynynen K. The combined concentric-ring and sector-vortex phased array for MRI guided ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control 1997;44(5):1157–1167 [Google Scholar]
- 22.Hutchinson EB, Hynynen K. Intracavitary ultrasound phased arrays for prostate thermal therapies: MRI compatibility and in vivo testing. Med Phys 1998;25(12):2392–2399 [DOI] [PubMed] [Google Scholar]
- 23.Dorr LN, Hynynen K. The effects of tissue heterogeneities and large blood vessels on the thermal exposure induced by short high-power ultrasound pulses. Int J Hyperthermia 1992;8(1):45–59 [DOI] [PubMed] [Google Scholar]
- 24.Yang R, Sanghvi NT, Rescorla FJ, Kopecky KK, Grosfeld JL. Liver cancer ablation with extracorporeal high-intensity focused ultrasound. Eur Urol 1993;23(Suppl 1):17–22 [DOI] [PubMed] [Google Scholar]
- 25.Kolios MC, Sherar MD, Hunt JW. Blood flow cooling and ultrasonic lesion formation. Med Phys 1996;23(7):1287–1298 [DOI] [PubMed] [Google Scholar]
- 26.Moritz AR, Henriques FC. Studies of thermal injury: II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol 1947;23(5):695–720 [PMC free article] [PubMed] [Google Scholar]
- 27.Crile G., Jr The effects of heat and radiation on cancers implanted on the feet of mice. Cancer Res 1963;23:372–380 [PubMed] [Google Scholar]
- 28.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984;10(6):787–800 [DOI] [PubMed] [Google Scholar]
- 29.Landry J, Marceau N. Rate-limiting events in hyperthermic cell killing. Radiat Res 1978;75(3):573–585 [PubMed] [Google Scholar]
- 30.Lele P. Threshold and mechanisms of ultrasonic damage to organized animal tissues. Presented at the Symposium on Biological Effects and Characterizations of Ultrasound Sources, Rockville, Md, 1977 [Google Scholar]
- 31.Hynynen K, Darkazanli A, Damianou CA, Unger E, Schenck JF. The usefulness of a contrast agent and gradient-recalled acquisition in a steady-state imaging sequence for magnetic resonance imaging-guided noninvasive ultrasound surgery. Invest Radiol 1994;29(10):897–903 [DOI] [PubMed] [Google Scholar]
- 32.Moonen CT, Madio DP, de Zwart JA, et al. MRI-guided focused ultrasound as a potential tool for control of gene therapy. Eur Radiol 1997;7:1165 [Google Scholar]
- 33.O’Neill BE, Li KC. Augmentation of targeted delivery with pulsed high intensity focused ultrasound. Int J Hyperthermia 2008;24(6):506–520 [DOI] [PubMed] [Google Scholar]
- 34.Vykhodtseva N, McDannold N, Hynynen K. Induction of apoptosis in vivo in the rabbit brain with focused ultrasound and Optison. Ultrasound Med Biol 2006;32(12):1923–1929 [DOI] [PubMed] [Google Scholar]
- 35.Coakley A. Acoustical detection of single cavitation events in a focused field in water at 1 MHz. J Acoust Soc Am 1971;49(3B):792–801 [Google Scholar]
- 36.Frizzell LA, Lee CS, Aschenbach PD, Borrelli MJ, Morimoto RS, Dunn F. Involvement of ultrasonically induced cavitation in the production of hind limb paralysis of the mouse neonate. J Acoust Soc Am 1983;74(3):1062–1065 [DOI] [PubMed] [Google Scholar]
- 37.ter Haar G, Daniels S, Eastaugh KC, Hill CR. Ultrasonically induced cavitation in vivo. Br J Cancer Suppl 1982;5:151–155 [PMC free article] [PubMed] [Google Scholar]
- 38.Smith NB, Hynynen K. The feasibility of using focused ultrasound for transmyocardial revascularization. Ultrasound Med Biol 1998;24(7):1045–1054 [DOI] [PubMed] [Google Scholar]
- 39.Xu Z, Ludomirsky A, Eun LY, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51(6):726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol 2006;32(1):115–129 [DOI] [PubMed] [Google Scholar]
- 41.Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol 1995;21(7):969–979 [DOI] [PubMed] [Google Scholar]
- 42.Hynynen K, Chung AH, Colucci V, Jolesz FA. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med Biol 1996;22(2):193–201 [DOI] [PubMed] [Google Scholar]
- 43.Hynynen K, Colucci V, Chung A, Jolesz F. Noninvasive arterial occlusion using MRI-guided focused ultrasound. Ultrasound Med Biol 1996;22(8):1071–1077 [DOI] [PubMed] [Google Scholar]
- 44.Umemura SI, Yumita N, Nishigaki R, Umemura K. Sonochemical activation of hematoporphyrin: a potential modality for cancer treatment. Proc IEEE Ultrason Symp 1989;2:955–960 [Google Scholar]
- 45.Siegel RJ, Cumberland DC, Myler RK, DonMichael TA. Percutaneous ultrasonic angioplasty: initial clinical experience. Lancet 1989;2(8666):772–774 [Published correction appears in Lancet 1989;2(8677):1468.] [DOI] [PubMed] [Google Scholar]
- 46.Rosenschein U, Bernstein JJ, DiSegni E, Kaplinsky E, Bernheim J, Rozenzsajn LA. Experimental ultrasonic angioplasty: disruption of atherosclerotic plaques and thrombi in vitro and arterial recanalization in vivo. J Am Coll Cardiol 1990;15(3):711–717 [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Hum Gene Ther 1996;7(11):1339–1346 [DOI] [PubMed] [Google Scholar]
- 48.Bednarski MD, Lee JW, Callstrom MR, Li KC. In vivo target-specific delivery of macromolecular agents with MR-guided focused ultrasound. Radiology 1997;204(1):263–268 [DOI] [PubMed] [Google Scholar]
- 49.Frenkel V, Oberoi J, Stone MJ, et al. Pulsed high-intensity focused ultrasound enhances thrombolysis in an in vitro model. Radiology 2006;239(1):86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone MJ, Frenkel V, Dromi S, et al. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thromb Res 2007;121(2):193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan Y, Laurencin CT. Fracture repair with ultrasound: clinical and cell-based evaluation. J Bone Joint Surg Am 2008;90(Suppl 1):138–144 [DOI] [PubMed] [Google Scholar]
- 52.Rutten S, Nolte PA, Korstjens CM, Klein-Nulend J. Low-intensity pulsed ultrasound affects RUNX2 immunopositive osteogenic cells in delayed clinical fracture healing. Bone 2009;45(5):862–869 [DOI] [PubMed] [Google Scholar]
- 53.Haar GT, Coussios C. High intensity focused ultrasound: past, present and future. Int J Hyperthermia 2007;23(2):85–87 [DOI] [PubMed] [Google Scholar]
- 54.Wu F. Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim Invasive Ther Allied Technol 2006;15(1):26–35 [DOI] [PubMed] [Google Scholar]
- 55.Rouvière O, Souchon R, Salomir R, Gelet A, Chapelon JY, Lyonnet D. Transrectal high-intensity focused ultrasound ablation of prostate cancer: effective treatment requiring accurate imaging. Eur J Radiol 2007;63(3):317–327 [DOI] [PubMed] [Google Scholar]
- 56.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89 [DOI] [PubMed] [Google Scholar]
- 57.Seip R, VanBaren P, Cain CA, Ebbini ES. Noninvasive real-time multipoint temperature control for ultrasound phased array treatments. IEEE Trans Ultrason Ferroelectr Freq Control 1996;43(6):1063–1073 [Google Scholar]
- 58.Curiel L, Chopra R, Hynynen K. In vivo monitoring of focused ultrasound surgery using local harmonic motion. Ultrasound Med Biol 2009;35(1):65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol 2010;195(3):W245–W252 [DOI] [PubMed] [Google Scholar]
- 60.Salomir R, Delemazure AS, Palussière J, Rouvière O, Cotton F, Chapelon JY. Image-based control of the magnetic resonance imaging-guided focused ultrasound thermotherapy. Top Magn Reson Imaging 2006;17(3):139–151 [DOI] [PubMed] [Google Scholar]
- 61.McDannold N, Tempany C, Jolesz F, Hynynen K. Evaluation of referenceless thermometry in MRI-guided focused ultrasound surgery of uterine fibroids. J Magn Reson Imaging 2008;28(4):1026–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higuchi N, Bleier AR, Jolesz FA, Colucci VM, Morris JH. Magnetic resonance imaging of the acute effects of interstitial neodymium:YAG laser irradiation on tissues. Invest Radiol 1992;27(10):814–821 [DOI] [PubMed] [Google Scholar]
- 63.Bleier AR, Jolesz FA, Cohen MS, et al. Real-time magnetic resonance imaging of laser heat deposition in tissue. Magn Reson Med 1991;21(1):132–137 [DOI] [PubMed] [Google Scholar]
- 64.Hindman JC. Proton resonance shift of water in the gas and liquid states. J Chem Phys 1966;44(12):4582–4592 [Google Scholar]
- 65.Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med 1995;34(6):814–823 [DOI] [PubMed] [Google Scholar]
- 66.Peters RD, Hinks RS, Henkelman RM. Ex vivo tissue-type independence in proton-resonance frequency shift MR thermometry. Magn Reson Med 1998;40(3):454–459 [DOI] [PubMed] [Google Scholar]
- 67.Kuroda K, Chung AH, Hynynen K, Jolesz FA. Calibration of water proton chemical shift with temperature for noninvasive temperature imaging during focused ultrasound surgery. J Magn Reson Imaging 1998;8(1):175–181 [DOI] [PubMed] [Google Scholar]
- 68.Pilatou MC, Stewart EA, Maier SE, et al. MRI-based thermal dosimetry and diffusion-weighted imaging of MRI-guided focused ultrasound thermal ablation of uterine fibroids. J Magn Reson Imaging 2009;29(2):404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daum DR, Hynynen K. A 256-element ultrasonic phased array system for the treatment of large volumes of deep seated tissue. IEEE Trans Ultrason Ferroelectr Freq Control 1999;46(5):1254–1268 [DOI] [PubMed] [Google Scholar]
- 70.Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 1998;24(2):275–283 [DOI] [PubMed] [Google Scholar]
- 71.Hynynen K, Clement GT, McDannold N, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med 2004;52(1):100–107 [DOI] [PubMed] [Google Scholar]
- 72.Hata T, Takahashi H, Watanabe K, et al. Magnetic resonance imaging for preoperative evaluation of breast cancer: a comparative study with mammography and ultrasonography. J Am Coll Surg 2004;198(2):190–197 [DOI] [PubMed] [Google Scholar]
- 73.Uematsu T, Yuen S, Kasami M, Uchida Y. Comparison of magnetic resonance imaging, multidetector row computed tomography, ultrasonography, and mammography for tumor extension of breast cancer. Breast Cancer Res Treat 2008;112(3):461–474 [DOI] [PubMed] [Google Scholar]
- 74.Schnall M. MR imaging evaluation of cancer extent: is there clinical relevance? Magn Reson Imaging Clin N Am 2006;14(3):379–381, vii [DOI] [PubMed] [Google Scholar]
- 75.Grobmyer SR, Mortellaro VE, Marshall J, et al. Is there a role for routine use of MRI in selection of patients for breast-conserving cancer therapy? J Am Coll Surg 2008;206(5):1045–1050; discussion 1050–1052 [DOI] [PubMed] [Google Scholar]
- 76.Hynynen K, Pomeroy O, Smith DN, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology 2001;219(1):176–185 [DOI] [PubMed] [Google Scholar]
- 77.Schmitz AC, Gianfelice D, Daniel BL, Mali WP, van den Bosch MA. Image-guided focused ultrasound ablation of breast cancer: current status, challenges, and future directions. Eur Radiol 2008;18(7):1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gombos EC, Kacher DF, Furusawa H, Namba K. Breast focused ultrasound surgery with magnetic resonance guidance. Top Magn Reson Imaging 2006;17(3):181–188 [DOI] [PubMed] [Google Scholar]
- 79.Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness— initial experience. Radiology 2003;227(3):849–855 [DOI] [PubMed] [Google Scholar]
- 80.Wu F, Wang ZB, Cao YD, et al. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer 2003;89(12):2227–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu F, Chen WZ, Bai J, et al. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 2001;27(8):1099–1106 [DOI] [PubMed] [Google Scholar]
- 82.Furusawa H, Namba K, Nakahara H, et al. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS). Breast Cancer 2007;14(1):55–58 [DOI] [PubMed] [Google Scholar]
- 83.Kennedy JE, Wu F, ter Haar GR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004;42(1-9):931–935 [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Fan WJ, Huang JH, et al. Comprehensive sequential interventional therapy for hepatocellular carcinoma. Chin Med J (Engl) 2009;122(19):2292–2298 [PubMed] [Google Scholar]
- 85.Fischer K, Gedroyc W, Jolesz FA. Focused ultrasound as a local therapy for liver cancer. Cancer J 2010;16(2):118–124 [DOI] [PubMed] [Google Scholar]
- 86.Civale J, Clarke R, Rivens I, ter Haar G. The use of a segmented transducer for rib sparing in HIFU treatments. Ultrasound Med Biol 2006;32(11):1753–1761 [DOI] [PubMed] [Google Scholar]
- 87.Zhu H, Zhou K, Zhang L, et al. High intensity focused ultrasound (HIFU) therapy for local treatment of hepatocellular carcinoma: role of partial rib resection. Eur J Radiol 2009;72(1):160–166 [DOI] [PubMed] [Google Scholar]
- 88.ter Haar G. High intensity ultrasound. Semin Laparosc Surg 2001;8(1):77–89 [PubMed] [Google Scholar]
- 89.Jolesz FA, Hynynen K, McDannold N, Freundlich D, Kopelman D. Noninvasive thermal ablation of hepatocellular carcinoma by using magnetic resonance imaging-guided focused ultrasound. Gastroenterology 2004;127(5 Suppl 1):S242–S247 [DOI] [PubMed] [Google Scholar]
- 90.Mikami K, Murakami T, Okada A, Osuga K, Tomoda K, Nakamura H. Magnetic resonance imaging-guided focused ultrasound ablation of uterine fibroids: early clinical experience. Radiat Med 2008;26(4):198–205 [DOI] [PubMed] [Google Scholar]
- 91.Gedroyc WM. New clinical applications of magnetic resonance-guided focused ultrasound. Top Magn Reson Imaging 2006;17(3):189–194 [DOI] [PubMed] [Google Scholar]
- 92.Wu F, Wang ZB, Chen WZ, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235(2):659–667 [DOI] [PubMed] [Google Scholar]
- 93.Holbrook AB, Santos JM, Kaye E, Rieke V, Pauly KB. Real-time MR thermometry for monitoring HIFU ablations of the liver. Magn Reson Med 2010;63(2):365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fennessy FM, Tuncali K, Morrison PR, Tempany CM. MR imaging-guided interventions in the genitourinary tract: an evolving concept. Magn Reson Imaging Clin N Am 2010;18(1):11–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thüroff S, Chaussy C, Vallancien G, et al. High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multicentric study. J Endourol 2003;17(8):673–677 [DOI] [PubMed] [Google Scholar]
- 96.Blana A, Murat FJ, Walter B, et al. First analysis of the long-term results with transrectal HIFU in patients with localised prostate cancer. Eur Urol 2008;53(6):1194–1201 [DOI] [PubMed] [Google Scholar]
- 97.Misraï V, Rouprêt M, Chartier-Kastler E, et al. Oncologic control provided by HIFU therapy as single treatment in men with clinically localized prostate cancer. World J Urol 2008;26(5):481–485 [DOI] [PubMed] [Google Scholar]
- 98.Uchida T, Ohkusa H, Yamashita H, et al. Five years experience of transrectal high-intensity focused ultrasound using the Sonablate device in the treatment of localized prostate cancer. Int J Urol 2006;13(3):228–233 [DOI] [PubMed] [Google Scholar]
- 99.Seip R, Ebbini ES. Noninvasive estimation of tissue temperature response to heating fields using diagnostic ultrasound. IEEE Trans Biomed Eng 1995;42(8):828–839 [DOI] [PubMed] [Google Scholar]
- 100.Maass-Moreno R, Damianou CA, Sanghvi NT. Noninvasive temperature estimation in tissue via ultrasound echo-shifts. Part II. In vitro study. J Acoust Soc Am 1996;100(4 Pt 1):2522–2530 [DOI] [PubMed] [Google Scholar]
- 101.Pernot M, Tanter M, Bercoff J, Waters KR, Fink M. Temperature estimation using ultrasonic spatial compound imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51(5):606–615 [PubMed] [Google Scholar]
- 102.Farny CH, Clement GT. Feasibility of ultrasound phase contrast for heating localization. J Acoust Soc Am 2008;123(3):1773–1783 [DOI] [PubMed] [Google Scholar]
- 103.Curiel L, Souchon R, Rouvière O, Gelet A, Chapelon JY. Elastography for the follow-up of high-intensity focused ultrasound prostate cancer treatment: initial comparison with MRI. Ultrasound Med Biol 2005;31(11):1461–1468 [DOI] [PubMed] [Google Scholar]
- 104.Madersbacher S, Kratzik C, Szabo N, Susani M, Vingers L, Marberger M. Tissue ablation in benign prostatic hyperplasia with high-intensity focused ultrasound. Eur Urol 1993;23(Suppl 1):39–43 [DOI] [PubMed] [Google Scholar]
- 105.Sokka SD, King R, Hynynen K. MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys Med Biol 2003;48(2):223–241 [DOI] [PubMed] [Google Scholar]
- 106.McDannold N, Ziso H, Assif B, et al. MRI-guided focused ultrasound (MRgFUS) system for thermal ablation of prostate cancer: Pre-clinical evaluation in canines [abstr]. In: Proceedings of the 17th Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2009 [Google Scholar]
- 107.Kinsey AM, Diederich CJ, Rieke V, et al. Transurethral ultrasound applicators with dynamic multi-sector control for prostate thermal therapy: in vivo evaluation under MR guidance. Med Phys 2008;35(5):2081–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chopra R, Baker N, Choy V, et al. MRI-compatible transurethral ultrasound system for the treatment of localized prostate cancer using rotational control. Med Phys 2008;35(4):1346–1357 [DOI] [PubMed] [Google Scholar]
- 109.Chopra R, Bronskill M, Haider M, Klotz L. Preliminary human evaluation of MRI-guided transurethral ultrasound therapy for the treatment of localized prostate cancer [abstr]. In: Proceedings of the 18th Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2010 [Google Scholar]
- 110.Wong PYH, Hata N, Szot Barnes A, et al. Displacement of neurovascular bundles before and after MRI-guided prostate brachytherapy [abstr]. In: Proceedings of the 15th Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2007 [Google Scholar]
- 111.Kelloff GJ, Choyke P, Coffey DS; Prostate Cancer Imaging Working Group Challenges in clinical prostate cancer: role of imaging. AJR Am J Roentgenol 2009;192(6):1455–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Afnan J, Tempany CM. Update on prostate imaging. Urol Clin North Am 2010;37(1):23–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol 2009;16(1):140–146 [DOI] [PubMed] [Google Scholar]
- 114.Meng X, He G, Zhang J, et al. A comparative study of fibroid ablation rates using radio frequency or high-intensity focused ultrasound. Cardiovasc Intervent Radiol 2010;33(4):794–799 [DOI] [PubMed] [Google Scholar]
- 115.Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology 2003;226(3):897–905 [DOI] [PubMed] [Google Scholar]
- 116.Ren XL, Zhou XD, Yan RL, et al. Sonographically guided extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: midterm results. J Ultrasound Med 2009;28(1):100–103 [DOI] [PubMed] [Google Scholar]
- 117.Shen SH, Fennessy F, McDannold N, Jolesz F, Tempany C. Image-guided thermal therapy of uterine fibroids. Semin Ultrasound CT MR 2009;30(2):91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol 2004;183(6):1713–1719 [Published correction appears in AJR Am J Roentgenol 2005;184(1):348.] [DOI] [PubMed] [Google Scholar]
- 119.Stewart EA, Rabinovici J, Tempany CM, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 2006;85(1):22–29 [Published correction appears in Fertil Steril 2006;85(4):1072.] [DOI] [PubMed] [Google Scholar]
- 120.Fennessy FM, Tempany CM. MRI-guided focused ultrasound surgery of uterine leiomyomas. Acad Radiol 2005;12(9):1158–1166 [DOI] [PubMed] [Google Scholar]
- 121.Fennessy FM, Tempany CM, McDannold NJ, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery—results of different treatment protocols. Radiology 2007;243(3):885–893 [DOI] [PubMed] [Google Scholar]
- 122.Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CM. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol 2007;110(2 Pt 1):279–287 [DOI] [PubMed] [Google Scholar]
- 123.Morita Y, Ito N, Hikida H, Takeuchi S, Nakamura K, Ohashi H. Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids - early experience. Eur J Obstet Gynecol Reprod Biol 2008;139(2):199–203 [DOI] [PubMed] [Google Scholar]
- 124.Leon-Villapalos J, Kaniorou-Larai M, Dziewulski P. Full thickness abdominal burn following magnetic resonance guided focused ultrasound therapy. Burns 2005;31(8):1054–1055 [DOI] [PubMed] [Google Scholar]
- 125.Morita Y, Ito N, Ohashi H. Pregnancy following MR-guided focused ultrasound surgery for a uterine fibroid. Int J Gynaecol Obstet 2007;99(1):56–57 [DOI] [PubMed] [Google Scholar]
- 126.Gavrilova-Jordan LP, Rose CH, Traynor KD, Brost BC, Gostout BS. Successful term pregnancy following MR-guided focused ultrasound treatment of uterine leiomyoma. J Perinatol 2007;27(1):59–61 [DOI] [PubMed] [Google Scholar]
- 127.Damianou C, Hynynen K. Focal spacing and near-field heating during pulsed high temperature ultrasound therapy. Ultrasound Med Biol 1993;19(9):777–787 [DOI] [PubMed] [Google Scholar]
- 128.Arora D, Minor MA, Skliar M, Roemer RB. Control of thermal therapies with moving power deposition field. Phys Med Biol 2006;51(5):1201–1219 [DOI] [PubMed] [Google Scholar]
- 129.Mougenot C, Salomir R, Palussière J, Grenier N, Moonen CT. Automatic spatial and temporal temperature control for MR-guided focused ultrasound using fast 3D MR thermometry and multispiral trajectory of the focal point. Magn Reson Med 2004;52(5):1005–1015 [DOI] [PubMed] [Google Scholar]
- 130.Kopelman D, Inbar Y, Hanannel A, et al. Magnetic resonance-guided focused ultrasound surgery using an enhanced sonication technique in a pig muscle model. Eur J Radiol 2006;59(2):190–197 [DOI] [PubMed] [Google Scholar]
- 131.Holt RG, Roy RA. Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med Biol 2001;27(10):1399–1412 [DOI] [PubMed] [Google Scholar]
- 132.Okada A, Morita Y, Fukunishi H, Takeichi K, Murakami T. Non-invasive magnetic resonance-guided focused ultrasound treatment of uterine fibroids in a large Japanese population: impact of the learning curve on patient outcome. Obstet Gynecol 2009;34(5):579–583 [DOI] [PubMed] [Google Scholar]
- 133.Lele PP. A simple method for production of trackless focal lesions with focused ultrasound: physical factors. J Physiol (Paris) 1962;160:494–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ram Z, Cohen ZR, Harnof S, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 2006;59(5):949–955; discussion 955–956 [DOI] [PubMed] [Google Scholar]
- 135.Marquet F, Pernot M, Aubry JF, et al. Non-invasive transcranial ultrasound therapy based on a 3D CT scan: protocol validation and in vitro results. Phys Med Biol 2009;54(9):2597–2613 [DOI] [PubMed] [Google Scholar]
- 136.Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am 2003;113(1):84–93 [DOI] [PubMed] [Google Scholar]
- 137.Hynynen K, McDannold N, Vykhodtseva N, et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg 2006;105(3):445–454 [DOI] [PubMed] [Google Scholar]
- 138.McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 2010;66(2):323–332; discussion 332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001;220(3):640–646 [DOI] [PubMed] [Google Scholar]
- 140.McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology 2006;241(1):95–106 [DOI] [PubMed] [Google Scholar]
- 141.Guerin C, Olivi A, Weingart JD, Lawson HC, Brem H. Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Invest New Drugs 2004;22(1):27–37 [DOI] [PubMed] [Google Scholar]
- 142.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron 2002;36(4):555–558 [DOI] [PubMed] [Google Scholar]
- 143.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 2004;30(7):979–989 [DOI] [PubMed] [Google Scholar]
- 144.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 2006;340(4):1085–1090 [DOI] [PubMed] [Google Scholar]
- 145.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A 2006;103(31):11719–11723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005;24(1):12–20 [DOI] [PubMed] [Google Scholar]
- 147.Bradley WG., Jr MR-guided focused ultrasound: a potentially disruptive technology. J Am Coll Radiol 2009;6(7):510–513 [DOI] [PubMed] [Google Scholar]
- 148.Jolesz FA, McDannold N. Current status and future potential of MRI-guided focused ultrasound surgery. J Magn Reson Imaging 2008;27(2):391–399 [DOI] [PubMed] [Google Scholar]
- 149.McDannold N, Tempany CM, Fennessy FM, et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology 2006;240(1):263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]