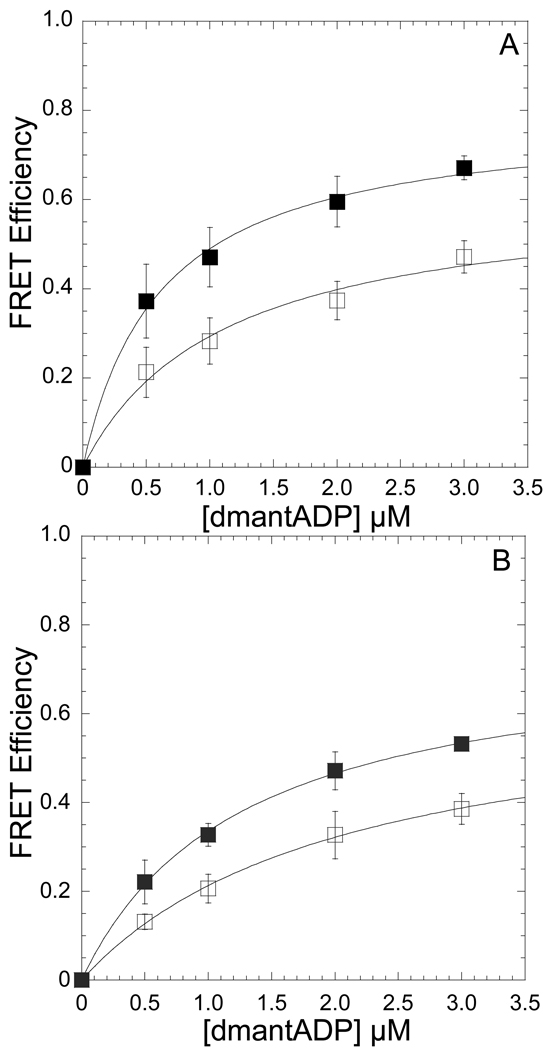
Figure 1. Conformation of the nucleotide-binding pocket as function of temperature monitored by FRET between dmantADP and MV FlAsH.
The emission of 0.5 µM MV FlAsH (A) or acto-MV FlAsH (B) was measured in the presence of increasing concentrations of dmantADP at 4 and 35 °C. The FRET efficiency was monitored by the enhancement in the acceptor fluorescence and the maximum FRET efficiency was determined by fitting the data to a hyperbolic binding function. Error bars represent the standard deviation from at least three separate experiments done with three different protein preparations.