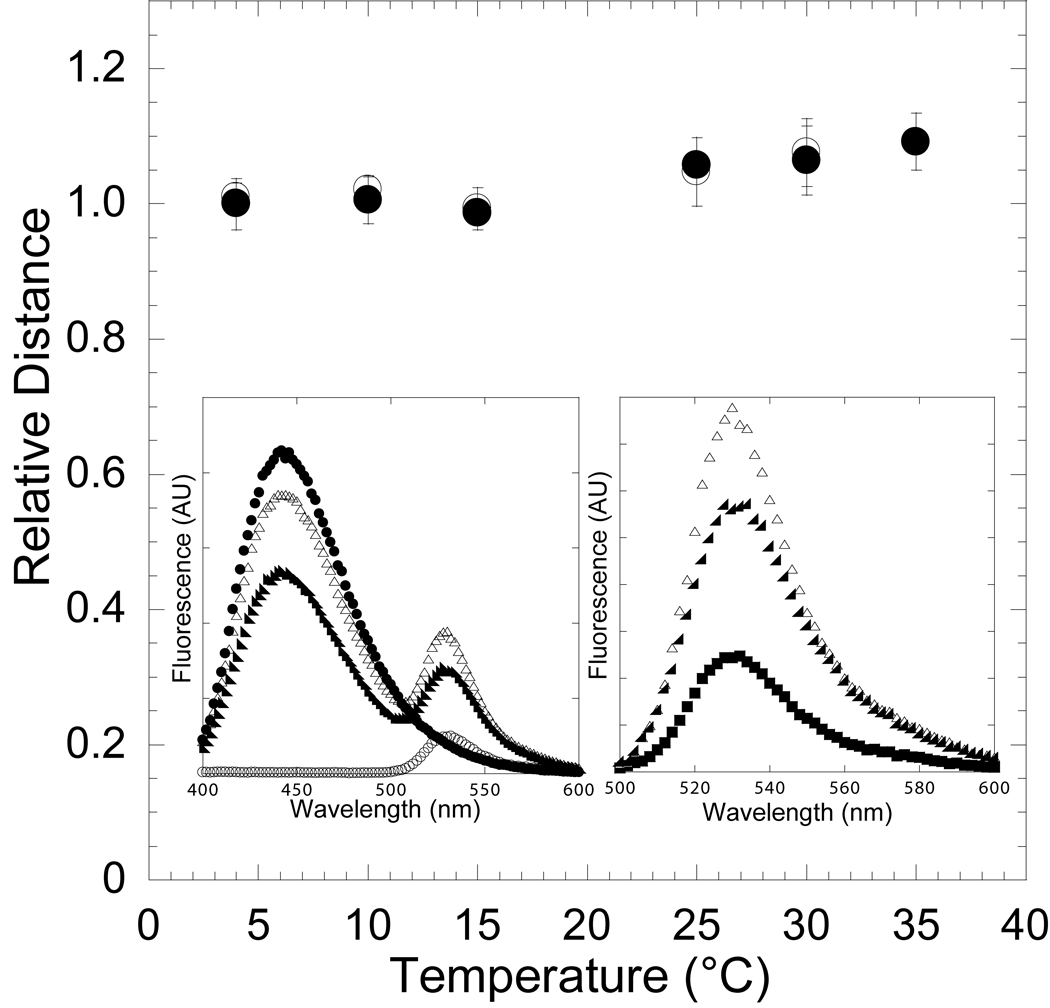
Figure 2. Reversibility of the temperature-dependent conformational change in the nucleotide-binding pocket in the presence of ADP.
The FRET of the acto-MV FlAsH:dmantADP complex was measured at 4, 10, 15, 25, 30, and 35 °C (closed circles) and then measured at 30, 25, 15, 10, and 4 °C (open circles) to demonstrate reversibility. The distance between the donor-acceptor pair was determined at each temperature and is shown relative to the distance at 4 °C. Error bars represent the standard deviation from at least three separate experiments done with three different protein preparations. The inset shows the fluorescence spectra of 0.5 µM acto-MV FlAsH in the presence of 3 µM dmantADP at 4 °C (closed circles) and 35 °C (closed triangles), acto-MV FlAsH in the presence of 3 µM unlabeled ADP (open circles), and 3 µM dmantADP (closed squares)(left panel). The MV FlAsH fluorescence after subtracting the dmantADP fluorescence component is shown for each sample (right panel).