Abstract
Background/Aims
The prevalence of gastric polyps, duodenal adenoma and duodenal cancer has been reported as being high among familial adenomatous polyposis (FAP) patients, but there have been no reports of this association in Korea. This study evaluated the prevalence of gastric and duodenal polyps and risk factors for duodenal neoplasm in FAP patients in Korea.
Methods
We reviewed both initial and follow-up endoscopic results from FAP patients. We also investigated the treatment modality of duodenal adenomas and analyzed the risk factors of duodenal neoplasms by logistic regression analysis.
Results
A total of 148 patients with FAP underwent esophagogastroduodenoscopy (EGD), and the fi ndings were as follows: gastric polyp 39.9% (fundic gland polyp 25.7% and gastric adenoma 14.2%), duodenal adenoma 15.5%, gastric cancer 2.7%, and duodenal cancer 0.7%. There were two cases of gastric cancer that developed from benign gastric polyps. There were progressions of duodenal adenomatosis during follow-up, and some degree of relapse occurred after endoscopic resection. Patients with gastric polyps showed a correlation with the occurrence of duodenal neoplasm (odds ratio, 2.814; p=0.024).
Conclusions
In Korean FAP patients, gastric cancer was detected more frequently, but fundic gland polyps, duodenal adenoma and duodenal cancer were detected less frequently than in Western patients. FAP patients with gastric polyps should undergo regular EGD, particularly for the early detection of duodenal neoplasia.
Keywords: Familial adenomatous polyposis, Duodenal cancer, Polyps, Adenomas, Gastric cancer
INTRODUCTION
Familial adenomatous polyposis (FAP) is characterized by the presence of at least one-hundred colorectal polyps and an earlyprogression to the colon cancer. Duodenum is the second most commonly affected site of polyp development in FAP patients.1 Duodenal polyps developed in FAP patients are adenomatous polyps, known as pre-cancerous lesions for duodenal adenocarcinoma.2 While prophylactic proctocolectomy has decreased the proportion of colorectal cancer as a cause of death in FAP patients, duodenal cancer has become a more important cause of death for these patients.3,4 Therefore, endoscopic surveillance is important for the early detection of duodenal cancer and can improve the prognosis of FAP patients.5
The stomach is also known as a common site of polyps in FAP patients. Fundic gland polyps are the most prevalent gastric lesions, followed by adenomatous polyps.6 In general, gastric cancer arises from adenomatous polyps via the adenomacarcinoma sequence, but several recent reports have shown that gastric adenocarcinoma can be developed from fundic gland polyp in FAP patients.7-9
In this study, we investigated the prevalence of gastric polyps and adenocarcinoma, the changing pattern through follow-up endoscopy and the precancerous possibility of gastric polyp in FAP patients. Also, we described the prevalence of duodenal polyps and adenocarcinoma, progression through follow-up endoscopy, and the factors related to the occurrence of duodenal neoplasm.
MATERIALS AND METHODS
1. Patients
From August 1978 to July 2006, the proctocolectomy was done in 171 FAP patients in Seoul National University Hospital, National Cancer Center of Korea, and Seoul National University Bundang Hospital. One hundred forty eight out of 171 patients (86.5%) underwent esophagogastroduodenoscopy (EGD) (GIF; Olympus, Tokyo, Japan) at initial diagnosis of FAP or during follow-up period. The 148 patients were enrolled in this study.
2. Investigation of EGD results
In 148 patients, we reviewed retrospectively the results of EGD to calculate the prevalence of gastric polyps, gastric adenocarcinoma, duodenal polyps and duodenal adenocarcinoma. Also, we reviewed the newly developed lesions and the cancerous changes of the lesions during follow-up by endoscopy. We investigated the distribution of histology in gastric and duodenal polyps and reviewed the treatment modality for duodenal adenoma.
3. The severity of duodenal adenomatosis
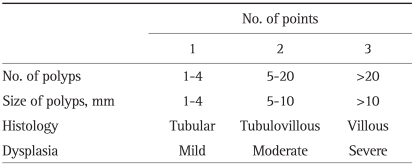
The severity of duodenal adenomatosis was staged according to the Spigelman classification (Table 1). In our hospitals, dysplasia was described just as low or high grade, so we considered low grade as mild dysplasia, Spigelman score 1 point, and high grade as severe dysplasia, Spigelman score 3 points. The change of Spigelman classification during follow-up was also investigated.
Table 1.
Spigelman Staging System of the Severity of Duodenal Adenomatosis
Stage 0, 0 points; Stage I, 1-4 points; Stage II, 5-6 points; Stage III, 7-8 points; Stage IV, 9-12 points.
4. Statistical analysis
The risk factors for the development of duodenal neoplasm were analyzed using the binary logistic regression analysis. All p-values were two-tailed, and a p-value<0.05 was considered statistically significant.
RESULTS
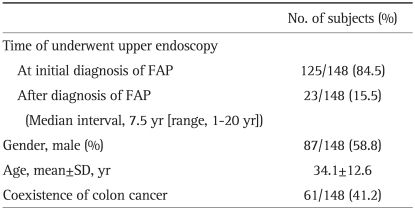
1. Baseline characteristics of patients
Among the 148 patients, 87 (58.8%) were male and 61 (41.2%) were female. The average age at initial diagnosis of FAP was 34.1 years, with extremes of age ranging from 13 to 77. Sixty one out of 148 patients (41.2%) had colon cancer at initial diagnosis of FAP. One hundred twenty five patients underwent EGD at initial diagnosis of FAP, but 23 patients did not undergo EGD at that time and underwent it during follow up. The median interval from initial diagnosis of FAP to the first endoscopy was 7.9 years (range, 1-0 years) (Table 2).
Table 2.
Baseline Characteristics of Patients with Familial Adenomatous Polyposis (FAP)
SD, standard deviation.
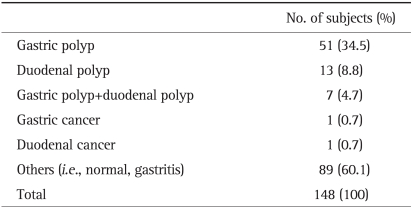
2. Results of the fi rst endoscopy
At the first endoscopy of 148 patients, gastric polyps were observed in 51 patients (34.5%), and duodenal polyps were detected in 13 patients (8.8%). Seven patients (4.7%) had both gastric polyps and duodenal polyps. One patient (0.7%) had a gastric cancer, and one patient (0.7%) had a duodenal cancer (Table 3).
Table 3.
Results of the First Endoscopy in Patients with Familial Adenomatous Polyposis
The patient with gastric cancer had not received initial EGD at the diagnosis of FAP and was diagnosed with early gastric cancer type IIc in the first endoscopy performed after 8 years at 43 years old. Subtotal gastrectomy was done and postoperative stage was T1N0M0. Background of adenoma was not observed in the lesion, but, there were multiple gastric adenomas around the lesion.
The patient with duodenal cancer underwent the first endoscopy at the diagnosis of FAP at 44 years old. The endoscopic finding showed there were several elevated, whitish adenomas from duodenal bulb to second portion. The biopsy result of the largest (2 cm) lesion was tubulovillous adenoma with low grade dysplasia. The Spigelman classification was stage III, 8 points. He underwent prophylactic proctocolectomy and duodenectomy at the same time. The final pathologic result after surgery was duodenal adenocarcinoma, well differentiated with stage T1N0M0 and the resection margin was clear. He has been followed up every six months, without recurrence for 24 months till now.
3. Results of the follow-up endoscopy
Of the 148 patients, 87 patients (58.8%) underwent follow-up endoscopy, the mean follow-up duration was 58 months (range, 6-240 months). Among 87 patients, 8 patients (9.2%) had newly developed gastric polyps, 10 patients (11.5%) had newly developed duodenal polyps, and 3 patients (3.4%) had newly developed gastric cancers. Fourteen patients (16.1%) had no changes in initially detected gastric or duodenal polyps. The other 52 patients had no polyps or mass in EGD.
Of the three patients with gastric cancer, one patient had an early gastric cancer IIb at 61 years old, another patient had an early gastric cancer IIc at 44 years old, and the other patient had an adenocarcinoma in cardia at 56 years old. The patient with early gastric cancer IIb received initial endoscopy at the diagnosis of FAP at 59 years old which showed gastric polyps in antrum and body, endoscopic mucosal resection was performed. The pathologic result was tubular adenomas with low and high grade dysplasia. One year later, recurrent gastric polyps were detected and the histopathologic examination showed tubular adenomas with low grade dysplasia. Duodenal adenoma was also observed and removed by polypectomy. One year later, a hyperemic lesion was observed in gastric body and biopsy result was adenocarcinoma. Total gastrectomy was done under the impression of early gastric cancer IIb and the stage was T1N0M0. Pathologically, it was well differentiated adenocarcinoma in the background of tubular adenoma, high grade. He has been disease free for 79 months till now. The patient with early gastric cancer IIc had no abnormality in initial endoscopy at the diagnosis of FAP at 42 years old, but two years later, early gastric cancer IIc was observed at endoscopy and removed by endoscopic mucosal resection. He has been disease free for 26 months till now. The patient with gastric adenocarcinoma in cardia had not received EGD at diagnosis of FAP at 32 years old. Nine years later, multiple fundic gland polyps in gastric body were observed in his first EGD. Three years later, no change was observed in endoscopy, but 12 years later after then, moderated differentiated gastric adenocarcinoma was detected in endoscopic biopsy. However, he was lost to follow-up, so it was not attested whether fundic gland polyps developed to adenocarcinoma.
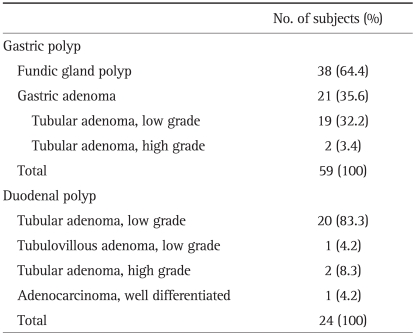
4. Histological distribution of gastric and duodenal polyps
Gastric polyps were detected in 59 patients (69.8%) in first or follow-up endoscopy and among them, fundic gland polyps in 38 patients (64.4%), gastric adenomas in 21 patients (35.6%) (tubular adenomas with low grade dysplasia in 19 patients and tubular adenomas with high grade dysplasia in 2 patients). No other types of polyp were observed.
Duodenal polyps were detected in 24 patients (16.2%), tubular adenomas with low grade dysplasia in 20 patients (83.3%), tubulovillous adenoma with low grade dysplasia in one patient (4.2%), tubular adenomas with high grade dysplasia in two patients (8.3%) and well differentiated adenocarcinoma in one patient (4.2%) (Table 4). All polyps in the duodenum were adenomas or adenocarcinoma.
Table 4.
Histological Distribution of Gastric and Duodenal Polyps
5. Classification by Spigelman staging system and changing patterns of duodenal adenomas during follow-up
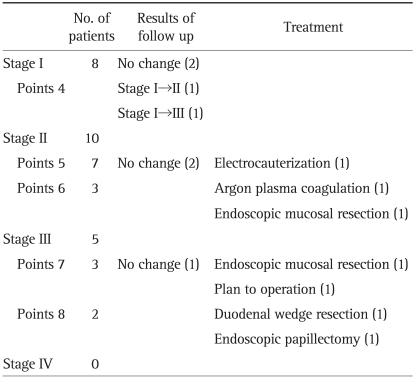
In 23 patients with duodenal adenoma, the Spigelman classification was: stage I, 8 patients (4 points, 8 patients); stage II, 10 patients (5 points, 7 patients; 6 points, 3 patients); stage III, 5 patients (7 points, 3 patients; 8 points, 2 patients); and stage IV, 0 patient (Table 5). And the location of lesions was as follows: duodenal second portion, 16 patients; bulb, 4 patients; transitional zone of bulb to second portion, 1 patient; bulb and second portion, 2 patients. Involvement of ampulla of Vater was seen in 2 patients.
Table 5.
Classification by Spigelman Staging and Treatment of Duodenal Adenomas
Among the patients of stage I, four patients underwent follow-up endoscopy and in them, no change was observed in two patients (mean follow-up period, 27 months), Spigelman stage was increased in other two patients. Among them, one patient obtained 5 points, stage II after 29 months. The other patient obtained 7 points, stage III after 14 months, and till 52 months, no change was observed. They were not treated in any modality.
In the patients of stage II, two patients with 5 points had no change in follow-up endoscopy (mean follow-up period, 72 months), were not treated. Other one patient with 5 points had bleeding at the lesion and endoscopic electrocauterization was done. One patient with 6 points had a 2.5 cm lesion, which was removed by endoscopic mucosal resection. The other patient with 6 points underwent argon plasma coagulation, but after 25 months, duodenal adenoma recurred like the previous lesion (stage II, 6 points).
In patients of stage III, one patient with 7 points was not treated, no change was observed in follow-up endoscopy after 48 months. The other patient with 7 points underwent endoscopic mucosal resection, and after 14 months, no abnormality was observed in follow-up endoscopy. Another patient with 7 points will receive surgical treatment because endoscopic resection has high risk of perforation due to broad base of the lesion. One patient with 8 points underwent endoscopic papillectomy, and after 38 months, the lesion was recurred but the stage was decreased as I, 4 points. The other patient with 8 points underwent duodenal wedge resection due to large sized polyp (2 cm).
6. Risk factors for the development of duodenal neoplasm
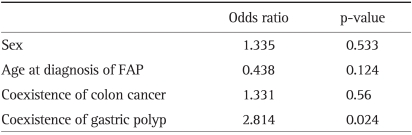
To evaluate the factors associated with the occurrence of duodenal neoplasm, many factors such as sex, age at diagnosis of FAP, coexistence of colon cancer and gastric polyp were analyzed by binary logistic regression analysis. The only statistically significant factor correlated with the occurrence of duodenal neoplasm was the coexistence of gastric polyp (odds ratio, 2.814; p=0.024) (Table 6).
Table 6.
Factors Associated with the Occurrence of Duodenal Neoplasm (Binary Logistic Regression Analysis)
The only statistically significant factor correlated with the occurrence of duodenal neoplasm was the coexistence of gastric polyp (odds ratio, 2.814; p=0.024).
FAP, familial adenomatous polyposis.
DISCUSSION
In this study, the incidence of gastric polyp was 39.9% (fundic gland polyp 25.7%, gastric adenoma 14.2%), duodenal adenoma was 15.5%, gastric cancer was 2.7% and duodenal cancer was 0.7%. During 58 months mean follow-up, the incidence of newly developed gastric polyp was 9.2% (8 patients), duodenal adenoma was 11.5% (10 patients), and gastric cancer was 3.4% (3 patients).
Fundic gland polyps are the most prevalent gastric polyps in FAP patients, the prevalence is about 50%.10,11 Recently, Lepistö et al.12 reported the prevalence of fundic gland polyp was about 60%. The prevalence of gastric adenoma was reported as 6-14%.13-15 However, the incidence of gastric cancer in Western FAP patients is not significantly different from the general population.16 On the other hand, Asian studies reported the increased prevalence of gastric cancer in FAP patients (4.2% in Korea and 2.1% in Japan).16,17 In this study, the incidence of fundic gland polyp are lower than Western reports, but that of gastric adenoma and gastric cancer is relatively high similar to previous Asian reports. In Korea, EGD by health screening is recommended for people age 40 and older in general population. In FAP patients, it is true that regular screening EGD is also necessary. There is no sufficient evidence to determine if screening EGD should be recommended to the people younger than 40 years, since this study showed that all three patients with cancer were above 40 years old.
The progression of gastric adenoma to adenocarcinoma is known as by the adenoma-carcinoma sequence, and recently, the cases that progress from fundic gland polyp to gastric cancer have been reported.7-9,18 Maybe, it is by the dysplasia-carcinoma pathway, Bianchi et al.19 reported the half of the fundic gland polyp in FAP patients showed dysplasia. In this study, one patient with fundic gland polyps had gastric adenocarcinoma in cardia after 15 years, but it is unclear that gastric adenocarcinoma was developed from fundic gland polyp because the surgical specimen is not available. Even if there was no pathological proof, the possibility that fundic gland polyp developed to adenocarcinoma in this case is present. Therefore, patients with gastric adenoma or fundic gland polyp should undergo regular EGD follow-up due to the risk of gastric cancer. Similar to Spigelman classification about the severity of duode-nal adenomatosis, a new classification of gastric polyps for the gastric cancer risk stratification is needed for the future.
According to the Western reports, duodenal adenomas can be found in 30-70% of FAP patients and the lifetime risk of these lesions approaches 100%.20 The incidence of duodenal cancer is rare, about 0.01-0.04% in general population,21 but FAP patients have a 100-330 fold higher risk of duodenal cancer compared with the general population.16 Bülow et al.15 reported the cumulative risk of developing duodenal cancer in FAP is about 4.5% at age of 57. In contrast to the Western reports, the incidence of duodenal adenoma and cancer was relatively low in our study. So, in Asian countries, the incidence of gastric cancer is higher and duodenal cancer is lower in FAP patients than in Western countries. It indicates that environmental as well as hereditary factors affect the cancer development.
The Spigelman staging classification is correlated with the risk of developing duodenal adenocarcinoma. Stage II, III, and IV diseases are associated with a 2.3%, 2.4%, and 36% risk of duodenal cancer, respectively.22 In our study, no case was observed that duodenal adenoma developed to adenocarcinoma. However, this study showed the stage progression in two patients from stage I to stage II and III and it is well known that duodenal adenomas progress slowly.23
Once duodenal cancer is developed, the prognosis is poor in spite of curative operation. Therefore, it is important to detect the lesion early by regular endoscopy. The recommended endoscopic follow up interval is every four years in stage 0, two-three years in stage I and II, six-twelve months in stage III, IV according to the Spigelman staging classification. It is recommended that patients with stage III are considered to be operated, those with stage IV should be operated.24 The beginning of endoscopic examination is recommended right after the diagnosis of FAP.25
In this study, three patients with stage II underwent endoscopic treatment; patients with stage III underwent endoscopic resection in one patient, endoscopic papillectomy in one patient, and duodenal wedge resection in one patient. Endoscopic techniques have developed in treatment of duodenal adenomatosis in FAP patients, so endoscopic snare excision, thermal ablation, argon plasma coagulation and photodynamic therapy are partly replacing open duodenotomy. Endoscopic resection showed the similar effect of decreasing Spigelman stage as duodenotomy.12 Thus, open duodenotomy is recommended only when endoscopic treatment is impossible.
However, recurrence rates of duodenal adenomatosis in FAP are high, about 50% in endoscopic treatment and 32-43% in duodenotomy.26-28 In our report, the stage II lesion in one patient removed by argon plasma coagulation was relapsed, and the stage III lesion in one patient removed by papillectomy was relapsed but stage was decreased. Therefore, follow-up endoscopy is absolutely necessary after endoscopic resection.
On the other hand, when the radical surgeries like pancreaticoduodenectomy or pylorus preserving pancreaticoduodenectomy were conducted, the recurrence rates of duodenal adenomatosis were zero or low.25,28,29 However, high recurrence rate was reported in patients who underwent extensive surgery for invasive duodenal cancer.30 Therefore, early detection of the lesions by the endoscopic follow-up is important, and they should be operated radically in case of high risk patients with duodenal cancer or severe dysplasia, extensive duodenal adenomatosis and Spigelman stage IV. In our study, duodenal adenocarcinoma was radically resected by duodenectomy because the lesion was detected early at T1N0M0. Followed up every six months, he has been remaining disease-free status. Because extensive surgeries like pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy have high complication rates, we should determine the treatment modality carefully case by case considering its pros and cons.
The only factor associated with the occurrence of duodenal neoplasm was the coexistence of gastric polyp. It means that endoscopy should be observed carefully and thoroughly down to the duodenum 2nd portion in patients with gastric polyps. Bianchi et al.19 presented that fundic gland polyp dysplasia is associated with large polyp size, increased severity of duodenal polyposis and antral gastritis. Further studies on the factors associated with the development of gastric polyp to cancer and the risk factors of the occurrence of duodenal neoplasm are required.
Our study has limitations because it was a retrospective study, and there were some cases of follow-up loss in the subjects. However, it shows the incidence of gastric polyp, gastric cancer, duodenal adenoma and adenocarcinoma, and changing patterns during follow up in 148 Korean patients with FAP. Furthermore, it represents the difference of incidence between Western and Asian countries. Our study demonstrates that coexistence of gastric polyp is associated with the occurrence of duodenal neoplasm for the first time. The use of side-viewing endoscopy, high resolution endoscopy and chromoendoscopy raises the detection rate of duodenal lesions,31 so it should be considered to use them in FAP patients who have high risk of duodenal neoplasm.
In conclusion, gastric cancer was detected higher, but fundic gland polyp, duodenal adenoma and duodenal cancer were detected lower than Western results. The patients with gastric polyps show the correlation with the occurrence of duodenal neoplasm, so regular EGD is necessary especially for the early detection of duodenal cancer.
ACKNOWLEDGEMENTS
Financial support: None.
Potential conflicts of interest: None.
References
- 1.Järvinen H, Nyberg M, Peltokallio P. Upper gastrointestinal tract polyps in familial adenomatosis coli. Gut. 1983;24:333–339. doi: 10.1136/gut.24.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spigelman AD, Talbot IC, Penna C, et al. Evidence for adenomacarcinoma sequence in the duodenum of patients with familial adenomatous polyposis. The Leeds Castle Polyposis Group (Upper Gastrointestinal Committee) J Clin Pathol. 1994;47:709–710. doi: 10.1136/jcp.47.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher MC, Phillips RK, Bulow S. Surveillance and management of upper gastrointestinal disease in familial adenomatous polyposis. Fam Cancer. 2006;5:263–273. doi: 10.1007/s10689-005-5668-0. [DOI] [PubMed] [Google Scholar]
- 4.Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149–1151. doi: 10.1016/s0140-6736(88)91962-9. [DOI] [PubMed] [Google Scholar]
- 5.Bülow S. Results of national registration of familial adenomatous polyposis. Gut. 2003;52:742–746. doi: 10.1136/gut.52.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–785. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 7.Zwick A, Munir M, Ryan CK, et al. Gastric adenocarcinoma and dysplasia in fundic gland polyps of a patient with attenuated adenomatous polyposis coli. Gastroenterology. 1997;113:659–663. doi: 10.1053/gast.1997.v113.pm9247488. [DOI] [PubMed] [Google Scholar]
- 8.Hofgärtner WT, Thorp M, Ramus MW, et al. Gastric adenocarcinoma associated with fundic gland polyps in a patient with attenuated familial adenomatous polyposis. Am J Gastroenterol. 1999;94:2275–2281. doi: 10.1111/j.1572-0241.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 9.Garrean S, Hering J, Saied A, Jani J, Espat NJ. Gastric adenocarcinoma arising from fundic gland polyps in a patient with familial adenomatous polyposis syndrome. Am Surg. 2008;74:79–83. [PubMed] [Google Scholar]
- 10.Sarre RG, Frost AG, Jagelman DG, Petras RE, Sivak MV, McGannon E. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut. 1987;28:306–314. doi: 10.1136/gut.28.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domizio P, Talbot IC, Spigelman AD, Williams CB, Phillips RK. Upper gastrointestinal pathology in familial adenomatous polyposis: results from a prospective study of 102 patients. J Clin Pathol. 1990;43:738–743. doi: 10.1136/jcp.43.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepistö A, Kiviluoto T, Halttunen J, Järvinen HJ. Surveillance and treatment of duodenal adenomatosis in familial adenomatous polyposis. Endoscopy. 2009;41:504–509. doi: 10.1055/s-0029-1214719. [DOI] [PubMed] [Google Scholar]
- 13.Debinski HS, Spigelman AD, Hatfield A, Williams CB, Phillips RK. Upper intestinal surveillance in familial adenomatous polyposis. Eur J Cancer. 1995;31A:1149–1153. doi: 10.1016/0959-8049(95)00171-e. [DOI] [PubMed] [Google Scholar]
- 14.Marcello PW, Asbun HJ, Veidenheimer MC, et al. Gastroduodenal polyps in familial adenomatous polyposis. Surg Endosc. 1996;10:418–421. doi: 10.1007/BF00191629. [DOI] [PubMed] [Google Scholar]
- 15.Bülow S, Björk J, Christensen IJ, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–386. doi: 10.1136/gut.2003.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Offerhaus GJ, Giardiello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–1982. doi: 10.1016/0016-5085(92)90322-p. [DOI] [PubMed] [Google Scholar]
- 17.Park JG, Park KJ, Ahn YO, et al. Risk of gastric cancer among Korean familial adenomatous polyposis patients. Report of three cases. Dis Colon Rectum. 1992;35:996–998. doi: 10.1007/BF02253505. [DOI] [PubMed] [Google Scholar]
- 18.Odze RD, Quinn PS, Terrault NA, et al. Advanced gastroduodenal polyposis with ras mutations in a patient with familial adenomatous polyposis. Hum Pathol. 1993;24:442–448. doi: 10.1016/0046-8177(93)90095-x. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:180–185. doi: 10.1016/j.cgh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Heiskanen I, Kellokumpu I, Järvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy. 1999;31:412–416. doi: 10.1055/s-1999-41. [DOI] [PubMed] [Google Scholar]
- 21.Sexe RB, Wade TP, Virgo KS, Johnson FE. Incidence and treatment of periampullary duodenal cancer in the U.S. veteran patient population. Cancer. 1996;77:251–254. doi: 10.1002/(SICI)1097-0142(19960115)77:2<251::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636–641. doi: 10.1136/gut.50.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49(3 Pt 1):358–364. doi: 10.1016/s0016-5107(99)70013-1. [DOI] [PubMed] [Google Scholar]
- 24.Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034–1043. doi: 10.1136/gut.2004.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morpurgo E, Vitale GC, Galandiuk S, Kimberling J, Ziegler C, Polk HC., Jr Clinical characteristics of familial adenomatous polyposis and management of duodenal adenomas. J Gastrointest Surg. 2004;8:559–564. doi: 10.1016/j.gassur.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Soravia C, Berk T, Haber G, Cohen Z, Gallinger S. Management of advanced duodenal polyposis in familial adenomatous polyposis. J Gastrointest Surg. 1997;1:474–478. doi: 10.1016/s1091-255x(97)80136-9. [DOI] [PubMed] [Google Scholar]
- 27.de Vos tot Nederveen Cappel WH, Järvinen HJ, Björk J, Berk T, Griffioen G, Vasen HF. Worldwide survey among polyposis registries of surgical management of severe duodenal adenomatosis in familial adenomatous polyposis. Br J Surg. 2003;90:705–710. doi: 10.1002/bjs.4094. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg. 2010;14:229–235. doi: 10.1007/s11605-009-1091-4. [DOI] [PubMed] [Google Scholar]
- 29.Ruo L, Coit DG, Brennan MF, Guillem JG. Long-term follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg. 2002;6:671–675. doi: 10.1016/s1091-255x(02)00045-8. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher MC, Shankar A, Groves CJ, Russell RC, Phillips RK. Pylorus-preserving pancreaticoduodenectomy for advanced duodenal disease in familial adenomatous polyposis. Br J Surg. 2004;91:1157–1164. doi: 10.1002/bjs.4527. [DOI] [PubMed] [Google Scholar]
- 31.Dekker E, Boparai KS, Poley JW, et al. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy. 2009;41:666–669. doi: 10.1055/s-0029-1214980. [DOI] [PubMed] [Google Scholar]