Figure 4.
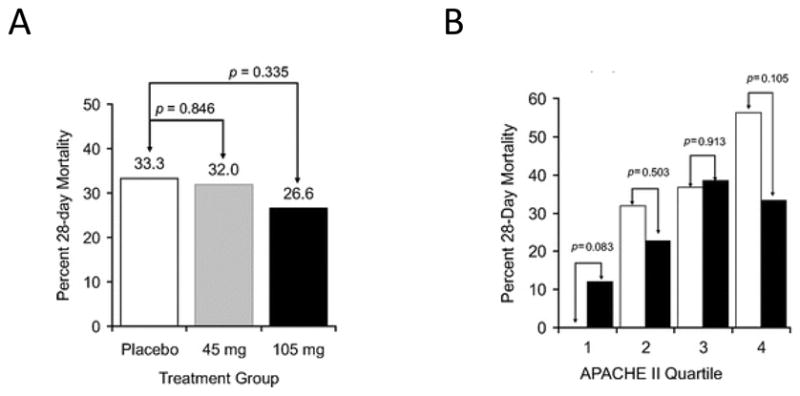
A. All-cause mortality at day 28 by treatment groups in the modified intention-to-treat population (total n = 293) from the Tidswell et al. study. Although mortality was lower in the eritoran 105mg group, it was not statistically different from placebo. B. All cause 28-day mortality in pre-specified subgroups defined by APACHE II quartiles treated with eritoran 105mg. Quartile I corresponds to an APACHE II score < 21, (n = 25, eritoran and n = 23, placebo); quartile 2, score 21–24 (n = 22, for both eritoran and placebo); quartile 3, score 25–28 (n = 26, eritoran and n = 19, placebo); and quartile 4, score > 28 (n = 21, eritoran and n = 32, placebo). Mortality in the treated group was lower than placebo for the 4th quartile with the highest APACHE II scores, but higher than placebo in the lowest quartile, with APACHE II < 21. Reproduced from [39]; with permission of Wolters Kluwer Health
