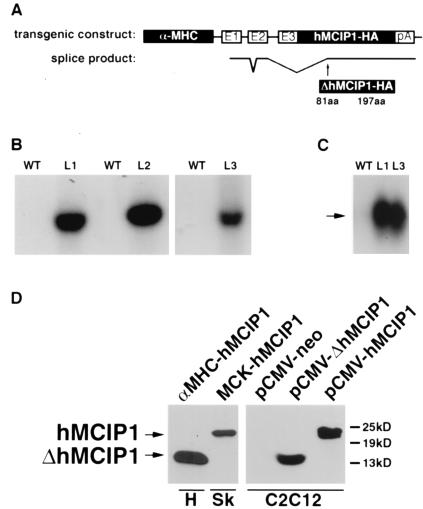
Figure 1.
Design and expression of the α−MHC-hMCIP1 transgene. (A) Schematic illustration of components of the transgene, including a 5.5-kb α−MHC promoter fragment with three noncoding exons (E1, E2, and E3) and intervening nontranscribed segments of the α−MHC gene, followed by a full-length human MCIP1 cDNA with a carboxyl terminal epitope tag (HA) and a polyadenylation (pA) signal from the human GH gene. The lower line illustrates the unexpected pattern of mRNA splicing observed in vivo, resulting in translation of a truncated protein (ΔhMICP1) initiated at amino acid 81 relative to the wild-type (WT) protein. (B) Southern blot of genomic DNA from WT mice and each of three lines (L1, L2, and L3) of α−MHC-hMCIP1 transgenic mice by using a probe specific to the human MCIP1 gene sequence. (C) Northern blot of heart mRNA from WT and two of the α−MHC-hMCIP1 transgenic mice lines (L1 and L3) by using a probe specific to human MCIP1 mRNA. Transgene expression was higher in the L2 line (data not shown). The arrow indicates the anomalously spliced 1.2-kb α−MHC-hMCIP1 transgene transcript (ΔhMCIP). Expression of the transgene was at least an order of magnitude higher than the endogenous 2.2-kb mMCIP1 transcript (data not shown). (D) Western blots probed to detect the hMCIP-HA protein in extracts from an L1 α−MHC-hMCIP1 transgenic heart (H) showing the 14-kDa truncated ΔhMCIP1 protein product. A positive control for comparison to a correctly spliced full length 24-kDa hMCIP1 protein was drawn from a skeletal muscle extract (Sk) from a line of transgenic mice expressing hMCIP1 under the control of the muscle creatine kinase promoter (MCK-hMCIP1). C2C12 cells were transfected with plasmids expressing the indicated products for size comparisons [pCMV-neo, pCMV-ΔhMCIP1 (amino acids 81 to 197) and pCMV-hMCIP1 (amino acids 1 to 197)].