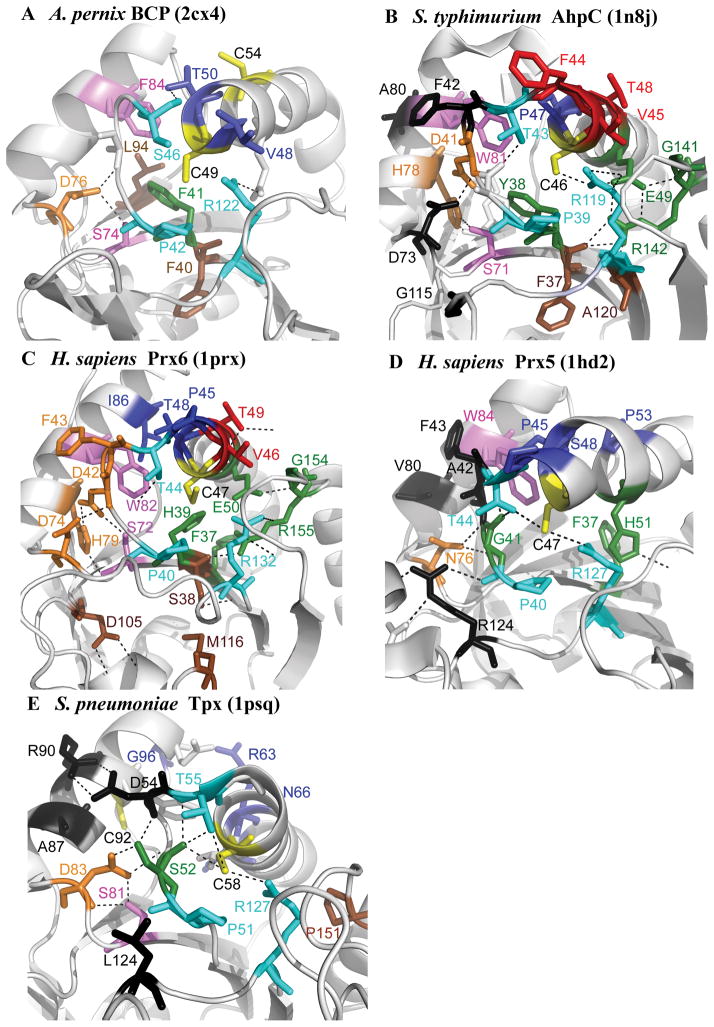
Figure 4. The location of conserved residues mapped to the structure for each subfamily.
Structures are shown for (A) Aeropyrum pernix BCP (2cx4), (B) Salmonella typhimurium AhpC (1n8j), (C) Homo sapiens Prx6 (1prx), (D) H. sapiens Prx5 (1hd2), and (E) Streptococcus pneumoniae Tpx (1psq). The CP and CR are in yellow, residues conserved across all Prx subfamilies in magenta and cyan, residues involved in forming the active site pocket of the reduced protein in green, residues found in the A-type interface in black, residues found in the B-type interface in red, residues involved in stabilizing the helix containing the CP in deep blue, residues forming a series of H-bonds between the key Thr residue and the region containing the A-type interface in orange, and conserved residues that do not fall into any of these groups in brown. Figure was made using Pymol (http://sourceforge.net/projects/pymol/).