Table 2.
Catalytic Asymmetric Conjugate Allylation of Methylidene Ketones in the Presence of (R,R,S)-L6.a
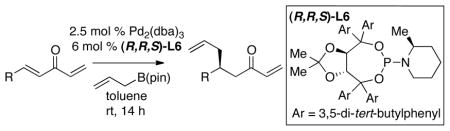 | ||||
|---|---|---|---|---|
| entry | product | regiob | % yieldc | erd |
| 1 | 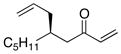 |
>100:1 | 59 | 93:7 |
| 2 | 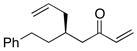 |
>100:1 | 79 | 94:6 |
| 3 | 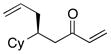 |
>100:1 | 57 | 95:5 |
| 4 | >100:1 | 91 | 91:9 | |
| 5 | 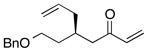 |
>100:1 | 53 | 91:9 |
| 6e | 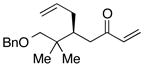 |
>100:1 | 81 | 96:4 |
| 7 | 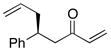 |
>100:1 | 80 | 95:5 |
| 8 | 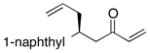 |
>100:1 | 37 | 96:4 |
| 9 | 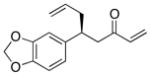 |
>100:1 | 76 | 95:5 |
Unless otherwise noted, the reaction was carried out at room temperature for 14 h and quenched with saturated NH4Cl(aq) or acetic acid.
Regioselectivity determined by uncalibrated GLC analysis in comparison to authentic materials.
Isolated yield after purification. Value is an average of two or more experiments.
Enantiomer ratio determined by GLC, SFC or HPLC analysis.
Experiment for 48 h and quenched with glacial acetic acid at 45 °C for 3 h.
