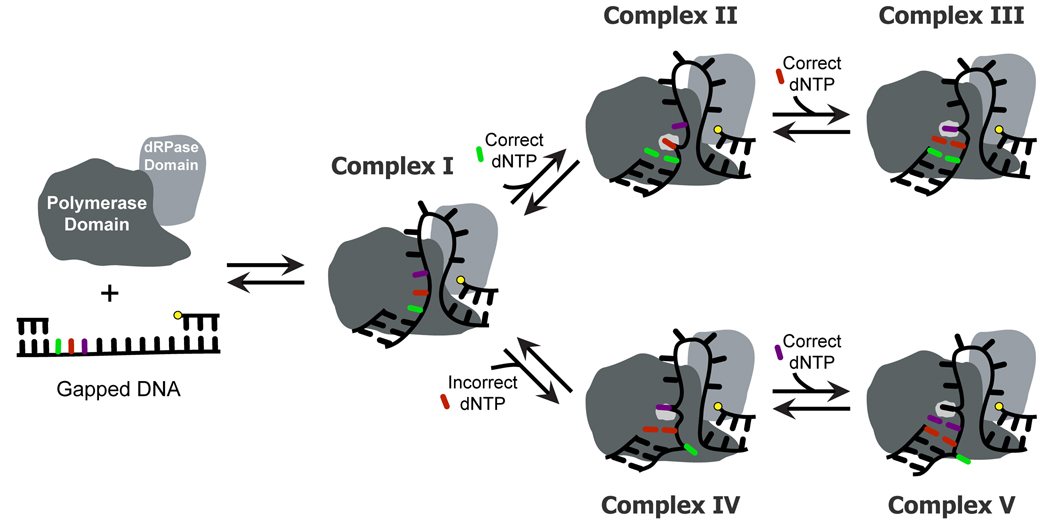
Figure 5. Model for long gap-filling DNA synthesis catalyzed by tPol λ.
Complex I shows the polymerase domain bound to the 3′-hydroxyl while the dRPase domain is bound to the 5′-phosphate (yellow dot) so that the single-stranded DNA template is looped out between the polymerase and dRPase domains. In the presence of a correct dNTP (Complex II), the first downstream template base is in the template “scrunching” binding pocket. Successive incorporations allow the downstream nucleotide that is immediately 5′ of the template base to reside in the scrunching pocket during each catalytic cycle (Complex III). In the presence of an incorrect dNTP that is complementary to the first downstream template base (Complex IV), the template base is looped out and the second downstream template base partially occupies the “scrunching” binding pocket. This complex would generate a frameshift deletion (Complex V).