Figure 1. N-glycan branching pathways and the galectin lattice.
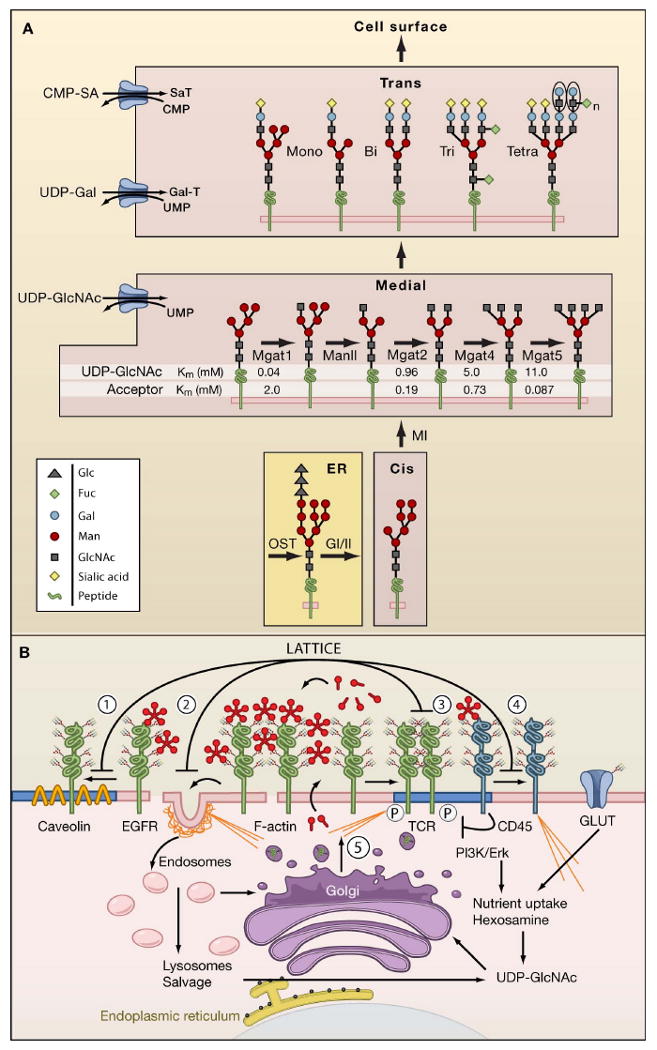
(A) N-glycan branching pathway. Oligosaccharyltransterase (OT) utilizes the pre-assembled donor Glc3Man9GlcNAc2-pp-dolichol to transfer the glycan to N-X-S/T motifs on glycoproteins in the endoplasmic reticulum (ER). Glycoproteins transit from the ER to cis, medial, and trans Golgi en route to the cell surface. The N-acetylglucosaminyltransferases enzymes, designated by their gene names (Mgat1, Mgat2, Mgat4, Mgat5) generate branched N-glycans that display a range of affinities for galectins. The Km values for Mgat1, Mgat2, Mgat4 and Mgat5 are indicated as measured in vitro for UDP-GlcNAc and acceptor glycoproteins.
(B) Dynamics of the galectin lattice. The glycocalyx is the thick carbohydrate layer surrounding the cell. Glycan structures generated in the Golgi differ in affinities for galectins. Galectins cross-link glycoprotein receptors and oppose (1) loss of epidermal growth factor receptors (EGFR) to Caveolin 1-positive microdomains, (2) coated-pit endocytosis, (3) precocious clustering of receptors, and (4) F-actin-mediated entry of T cell receptor (TCR) into and exit of CD45 from ganglioside GM1-positive microdomains (blue). (5) Nutrient supply and growth signaling increase membrane remodeling, regulate metabolite flux through the hexosamine pathway to UDP-GlcNAc and N-glycan branching (Golgi) on receptors to promote surface retention by the galectin lattice.
