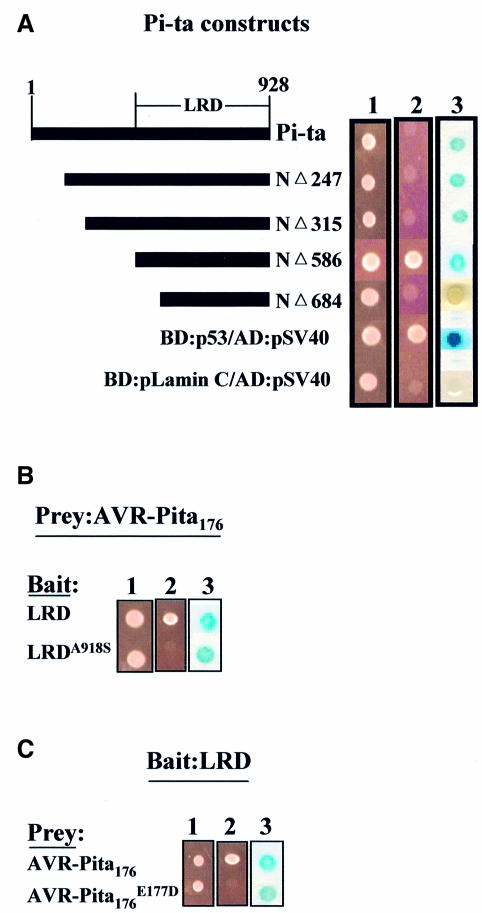
Fig. 4. AVR-Pita176 interacts specifically with the Pi-ta LRD in the yeast two-hybrid system. (A) Mapping the interaction domain of the Pi-ta protein using the yeast two-hybrid system. The diagram (left) depicts the Pi-ta protein, the LRD region and a series of deletion constructs of Pi-ta. (1) YRG2 yeast cells expressing both bait and prey fusions were grown on yeast synthetic minimal (SD, Stratagene) liquid medium with omission of leucine and tryptophan (SD-LT), and (2) on yeast SD medium with omission of leucine, histidine and tryptophan for examination of HIS3 reporter gene (–LHT). (3) Yeast cells expressing both bait and prey fusions were grown on yeast SD-LT plates and assayed for lacZ activity as described in Materials and methods. Color development is shown after 24 h. AVR-Pita176 was cloned as an AD fusion (in pAD-GAL4) and each Pi-ta deletion construct was cloned as a BD fusion (in pBD-GAL4 Cam). The positive control was YRG2 cells containing p53 and pSV40 fusion constructs, which express proteins that interact in vivo, and the negative control was YRG2 cells containing pLamin C and pSV40 fusion constructs, which express proteins that do not interact in vivo (Stratagene). (B and C) Specificity of interaction of AVR-Pita176 with LRD polypeptide. Single amino acid substitutions that inactivate either LRD or AVR-Pita 176 in vivo eliminated growth in the absence of histidine and slowed color development with the lacZ reporter (referred to as an impaired physical interaction). LRD refers to the NΔ586 deletion of the Pi-ta protein from (A) above. LRDA918S refers to LRD containing S substituted for A at position 918 of the Pi-ta protein. avr-pita176E177D refers to the putative processed polypeptide that no longer confers avirulence due to an E to D substitution at residue 177 of AVR-Pita223. Similar expression of each BD fusion protein in yeast was verified by western blots.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.