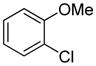
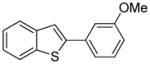
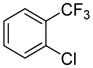
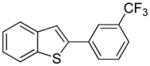
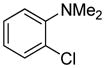
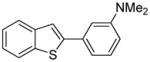
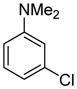
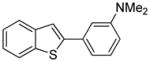
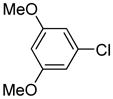
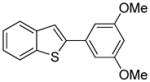
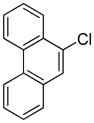
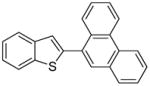
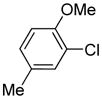
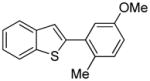
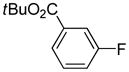
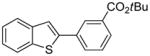
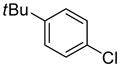
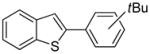
Table 1.
Arylation scope with respect to aryl halidesa
Aryl halide (1.6–2.5 equiv), benzothiophene (1 equiv), 0.5 mmol scale. Yields are isolated yields. See Supporting Information for details.
tert-Butyl-3-bromobenzoate used.
Isomer mixture; m/p ratio 1/1.2.