Table 2.
Arylation scope with respect to heterocycles and arenesa
 | |||
|---|---|---|---|
| entry | arene | product | yield, % |
| 1 |
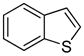
|
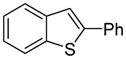
|
86 72b 85c |
| 2 |
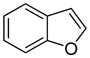
|
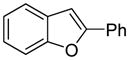
|
81 75d |
| 3 |
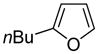
|
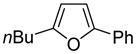
|
80 68b |
| 4 |
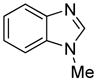
|
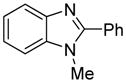
|
91 81b |
| 5 |
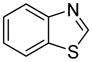
|
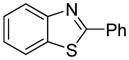
|
72 |
| 6 |
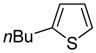
|
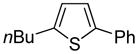
|
80 74b |
| 7 |
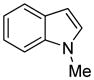
|
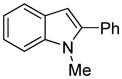
|
90 |
| 8e |
|
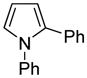
|
78 |
| 9 |
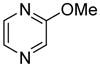
|
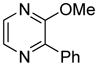
|
55 |
| 10 |
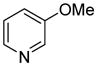
|
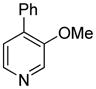
|
71 |
| 11 |
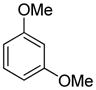
|
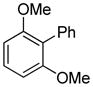
|
95 |
| 12 |
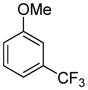
|
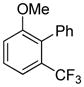
|
81 |
Chlorobenzene (1.3–2 equiv), arene or heterocycle (1 equiv), 0.5 mmol scale. Yields are isolated yields. See Supporting Information for details.
Lithium dicyclohexylamide base.
PhF used instead of PhCl.
LiNiPr2 base.
Phenylpyrrole (2 equiv), PhCl (1 equiv).
