Table 1.
Optimization of the triple aldol reaction.
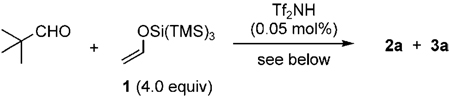 | |||||
|---|---|---|---|---|---|
| Entry | Solvent |
T [°C] |
Additive [mol%] |
Yield of 2a [%][a] |
Yield of 3a [%][a,b] |
| 1 | CH2Cl2 | 0 to 40 | none | 75 | 0 |
| 2 | hexanes | 23 to 65 | none | <5 | 0 |
| 3 | toluene | −78 to 80 | none | <5 | 0 |
| 4 | PhCl | 0 to 50 | none | 60 | <5 |
| 5 | C8F18 | 0 to 60 | none | 20 | <5 |
| 6 | CH2Cl2 | 0 to 23 | ICH2CH2I (10) | 37 | 31 |
| 7 | CH2Cl2 | −78 to 23 | MeI (10) | 71 | 13 |
| 8 | CH2Cl2 | −40 to 0 | PhI (10) | 30 | 52 |
| 9 | CH2Cl2 | −40 to 23 | 1,2-C6H4I2 (10) | 49 | 27 |
| 10 | CH2Cl2 | −40 to 23 | 2-I-py (10) | <5 | 0 |
| 11[c] | CH2Cl2 | −40 to 23 | I2 (5.0) | 65 | 7 |
| 12[c] | CH2Cl2 | −40 to 23 | PhI (10) | <5 | 85 |
| 13[c] | CH2Cl2 | −40 to 0 | PhI (10) | 64 | 32 |
| 14[c] | CH2Cl2 | 0 | PhI (10) | 29 | 51 |
| 15[c] | CH2Cl2 | 23 | PhI (10) | 15 | 47 |
| 16[c] | CH2Cl2 | −40 to 0 | PhI (2.0) | 12 | 77 |
| 17[c] | CH2Cl2 | −40 to 0 | PhI (0.5) | 46 | 26 |
Yield of combined isolated diastereomers.
d.r.=87:10:2:<1 as determined by crude 1H NMR spectroscopic and HPLC analyses.
5.0 equivalents of 1.
