Table 2.
Scope of the aldehyde substrate in the triple-aldol reaction with 1.
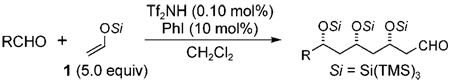 | ||||
|---|---|---|---|---|
| Entry | Product | Yield [%][a] | d.r.[b] | |
| 1 | 3b | 84 | 79:10:9:<2 | |
| 2 | 3c | 87 | 81:9:8:<2 | |
| 3 | 3d | 75 | 81:9:8:<2 | |
| 4 | 3e | 87 | 71:14:12:2 | |
| 5[c] | 3f | 89 | 87:8:3:2 | |
| 6 | 3g | 54 | –[d] | |
| 7[e] | 3h | 57 | –[d] | |
Yield of combined isolated diastereomers, unless otherwise noted.
The diastereomeric ratios were determined by crude 1H NMR spectroscopic and HPLC analyses.
0.2 mol% Tf2NH was used.
this yield is for the diastereomer shown only;
10 mol% 1-iodo-3,3-dimethyl-1-butyne was employed in this reaction. Cy=cyclohexyl, Bn=benzyl, TBS=tert-butyldimethylsilyl, TIPS=triisopropylsilyl.
