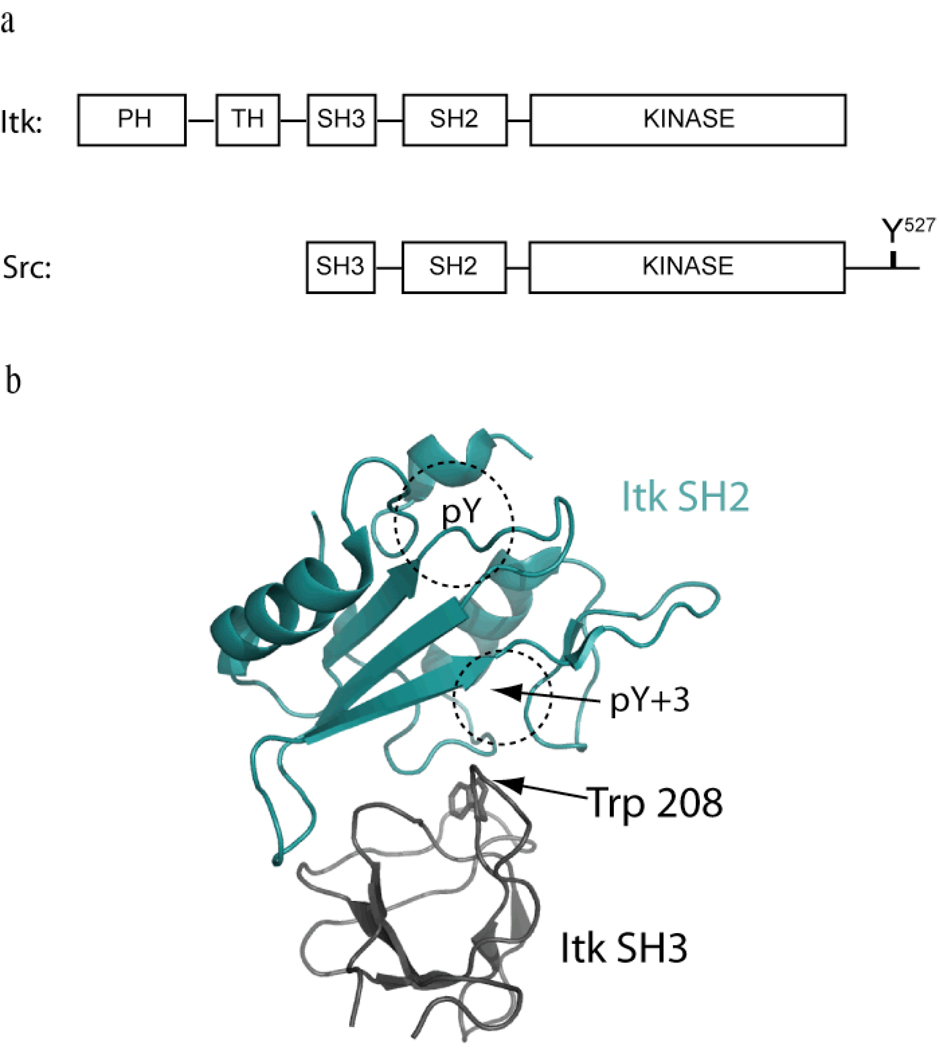
Figure 1. Negative regulation of Itk; domain structure differences with Src suggest an alternative mode of regulation.
(a) Domain structures of Itk and Src. Both kinases contain the SH3-SH2-Kinase domain cassette but differ at both the amino- and carboxy termini. Itk contains the Pleckstrin homology/Tec homology (PH-TH) region that is absent in Src. Src contains the negative regulatory tail region lacking in Itk. Y527 in the Src tail gets phosphorylated and binds in an intramolecular fashion to the Src SH2 domain to negatively regulate Src kinase activity (15). Itk is not regulated in the same manner but the SH3 and SH2 domains associate with each other in an intermolecular sense (18). (b) The three-dimensional structure of the Itk SH3/SH2 complex (pdb: 2K79) has been solved (16). The Itk SH2 domain (shown in cyan) binds to the ligand-binding pocket on the Itk SH3 domain (shown in black). Trp 208 is located in the SH3 binding pocket and mediates binding of the SH3 domain to both the Itk SH2 domain and classical proline-rich ligands. The classical phospholigand binding pockets (pY and pY+3) are labeled on the SH2 domain. The SH3 binding site on the SH2 domain partially overlaps with phospholigand binding on SH2.