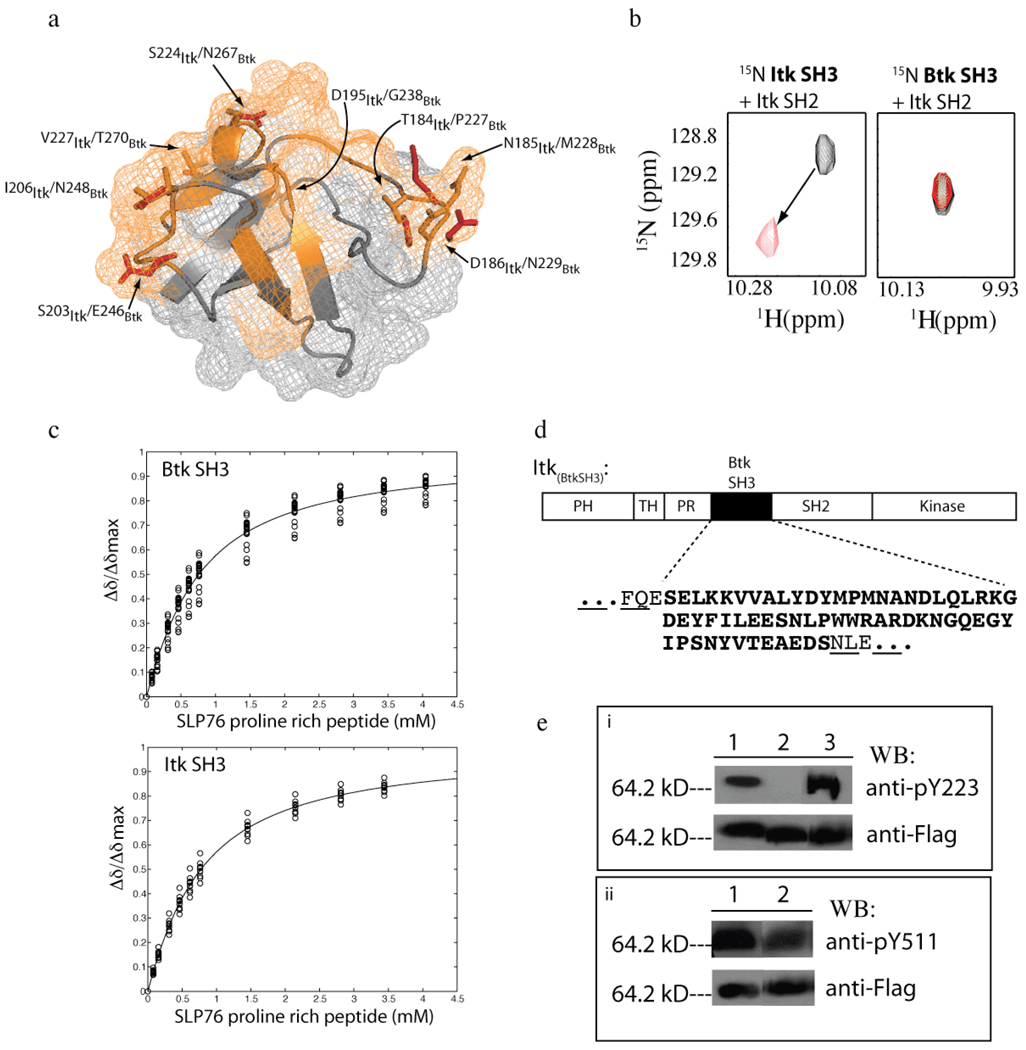
Figure 2. Specific disruption of the intermolecular SH3/SH2 interaction.
(a) The Itk SH3 and Btk SH3 domain structures are superimposed (only labeled side chains from the Btk SH3 domain are shown for clarity). The Itk SH3/SH2 interaction surface is indicated in orange on the SH3 structure (18). Within this binding surface, only eight residues are not conserved between Itk and Btk. Each of the non-conserved pairs of residues are labeled and indicated on the structure (orange are Itk side chains and red are Btk side chains). (b) Select region of heteronuclear single quantum coherence (HSQC) spectra of 15N-labeled SH3 domains (from either Itk (left) or Btk (right)) indicating the extent of chemical shift perturbations associated with binding to unlabeled Itk SH2 domain. For both, the black spectrum is 15N labeled SH3 protein alone and the red spectrum has been acquired following addition of equimolar unlabeled Itk SH2 domain in each case. The arrow indicates the change in resonance frequency for a representative peak upon addition of Itk SH2 to 15N labeled Itk SH3 domain. Addition of unlabeled Itk SH2 domain to 15N labeled Btk SH3 domain results in no change in chemical shift values. This result confirms the absence of an interaction between Itk SH2 and Btk SH3. (c) Concentration dependence of the normalized 1H chemical shift (Δδ/Δδmax) for representative residues of 15N labeled Btk SH3 domain (top) and 15N labeled Itk SH3 domain (bottom) upon titration of the Slp76 derived proline-rich peptide. The titration data were acquired and analyzed as previously described (21). (d) Design of the Itk(BtkSH3) protein. The Itk SH3 sequence within full-length Itk is replaced with the SH3 sequence of Btk. The sequence of the Btk SH3 domain is shown in bold below the domain map of Itk and the surrounding Itk residues within the resulting Itk(BtkSH3) are non-bold and underlined. (e) Western blot analysis using phosphotyrosine specific antibodies to the autophosphorylation site in the SH3 domain (Y180 for Itk/Y223 for Btk) and the activation loop tyrosine (Y511). (i) Lane 1: wild-type full-length Itk, lane 2: full-length, mutant Itk(Y180F) and lane 3: Itk(BtkSH3) were expressed as fusions with the flag peptide and purified from insect cells, resolved by SDS-PAGE, transferred to PDVF membrane and blotted with the Btk antibody to pY223 (Btk numbering; this tyrosine corresponds to Y180 in Itk) to detect the autophosphorylation status in the SH3 domain (top panel). Cross reactivity of the Btk pY223 antibody for Itk pY180 has been previously demonstrated (9). (ii) Lane 1: wild type full length Itk, lane 2: Itk(BtkSH3) were prepared as described above and blotted with the anti-pY551 antibody to detect the activation loop phosphorylation status (top panel). Itk protein levels are indicated in the bottom panel for both (i) and (ii).