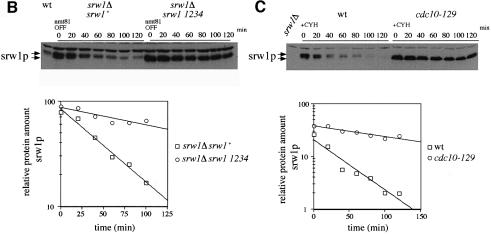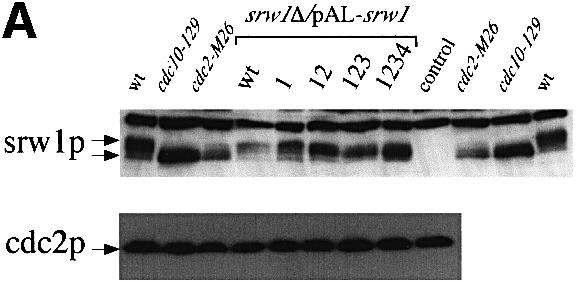
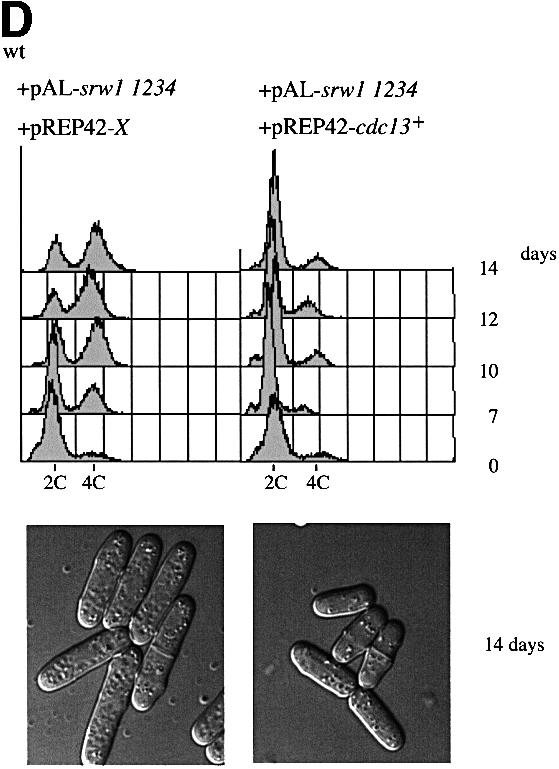

Fig. 7. srw1p stability is regulated by phosphorylation on Cdk consensus sites. (A) Samples were taken from exponentially growing srw1Δ cells carrying pAL-srw1 with no (wt), single (1), double (12), triple (123) or quadruple (1234) mutations in Cdk consensus sites or empty vector (pAL-X). Extracts from wild-type cells, cdc10-129 blocked cells and cdc2-M26 blocked cells were loaded as controls. Extracts were western blotted and probed with anti-srw1p and anti-cdc2p antibodies. (B) srw1 was shut off by adding thiamine (+T) to srw1Δ cells expressing wild-type srw1 (srw1Δ nmt81-srw1+) or quadruple mutant srw1 (srw1Δ nmt81-srw1 1234) and cells were incubated at 30°C for the times indicated. Extracts from wild-type cells were loaded to show the endogenous level of srw1p. Extracts were western blotted and probed with anti-srw1p antibody, the srw1p protein was quantified with NIH image analyser. (C) Wild-type cells and cdc10-129 cells were incubated for 4 h at 36°C, cycloheximide (CYH) was added, and cells were incubated at 36°C for the times indicated. (D) Flow cytometry and morphology of wild-type cells co-expressing quadruple mutant srw1 (pAL-srw1 1234) and either pREP42-X or pREP42-cdc13+. Cells were incubated for the number of days indicated at 30°C in minimal medium in the absence of thiamine. (E) cdc13 expression was turned off by adding thiamine to cdc13Δ REP41-cdc13+ and cdc13Δ REP41-cdc13+ srw1:: srw1 1234 strains and cells were further incubated at 32°C for the time indicated in minutes. Extracts were western blotted and probed with anti-cdc13p, anti-srw1p and anti-cdc2p antibodies.