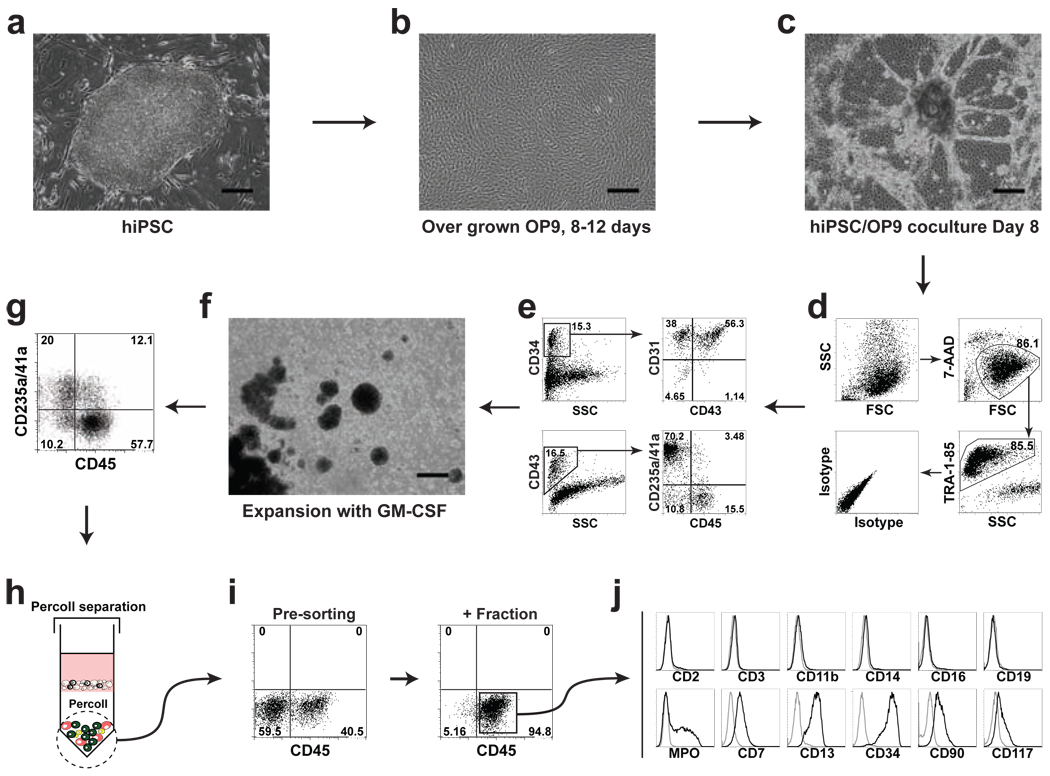
Figure 1.
Schematic diagram of the protocol used to obtain multipotent lin−CD34+CD43+CD45+ progenitors from human pluripotent stem cells. (a) Undifferentiated hiPSCs (iPS(Foreskin)-1) cocultured with (b) overgrown OP9. (c) After 8 days of coculture differentiated blood-forming hPSC colonies with radial sac-like structures are formed. (d) and (e) Typical flow cytometric analysis of differentiated iPS(Foreskin)-1 cells collected after 8 days of OP9 cocluture shows that hiPSC-derived CD34+ cells consist of CD31+CD43− endothelial cells, CD43+ hematopoietic cells, and CD31−CD43− mesenchymal cells. Three major subsets of hematopoietic progenitors could be defined within CD43+ population: CD235a/41a+ erythro-megakaryocytic progenitors, and CD235a/CD41a−CD45− and CD235a/CD41a−CD45+ multipotent progenitors. For the analysis, live (7-AAD−) and human (TRA-1-85+) cells are gated. (f) When differentiated hiPSCs are collected on day 8 of OP9 coculture by enzymatic digestion and cultured in nonadherent conditions in the presence of GM-CSF, cells spontaneously form large aggregates with hematopoietic cells released from these aggregates into suspension. (g) Typical flow cytometric analysis of cells cultured with GM-CSF for 2 days shows a marked expansion of the CD45+ population of hematopoietic progenitors within the CD43+ population. (h) After Percoll separation and (i) magnetic sorting a population of CD45+ progenitors with greater than 90% purity can be obtained. (j) Isolated CD45+ progenitors do not express major lineage markers, but retain expression of CD34, CD90, and CD177 markers characteristic of primitive hematopoietic cells. Scale bars=300µm.