Abstract
Study design
Retrospective chart review.
Objective
To define the temporal course of weight gain in persons with new spinal cord injury (SCI), and to identify predictors of weight gain in this population.
Setting
A United States Department of Veterans Affairs (VA) SCI Unit.
Methods
A retrospective chart review in a VA SCI Unit was conducted. Participants (n = 85) included all persons with new SCI completing initial rehabilitation at the center between 1998 and 2006. Outcome measures were mean change in body mass index (BMI) between rehabilitation admission and final follow-up, time of greatest BMI change, and distribution of participants by BMI classification. These measures were also examined relative to SCI level, American Spinal Injury Association Impairment Scale (AIS) grade, primary mode of mobility, and age at rehabilitation admission.
Results
Mean BMI increased by 2.3 kg/m2 between rehabilitation admission (mean 45 days post-injury) and final follow-up (mean 5 years post-injury). The distribution of participants shifted from lower BMI classifications at rehabilitation admission to higher BMI classifications at final follow-up. For participants transitioning from normal to overweight or obese, the greatest increase occurred during the first year after acute rehabilitation. Neurological level, impairment category, primary mode of mobility, and age at rehabilitation admission did not significantly predict BMI change. BMI at rehabilitation admission correlated significantly with BMI at final follow-up (P < 0.0005).
Conclusions
These findings confirm a significant increase in BMI after new SCI and suggest that persons with new SCI are at greatest weight gain risk during the first year following acute rehabilitation.
Keywords: Spinal cord injury, Obesity
Introduction
Obesity is the most prevalent nutrition problem in the United States; an estimated 64% of US adults are either overweight or obese, with overweight defined as body mass index (BMI) 25 kg/m2 and obesity as BMI 30 kg/m2.1 Adults with disability are more likely to be obese than those without disability.2 Two studies of US veterans found that two-thirds of spinal cord injury (SCI) persons are overweight or obese by the above BMI criteria.1,3
Defining obesity in SCI persons is controversial because of the muscle atrophy and bone mineral loss that leads to fat-free mass loss and to lower BMIs, which can underestimate the gain in fat mass. Although BMI is a widely accepted marker of obesity and cardiovascular risk in able-bodied populations, the relationship of BMI to cardiovascular risk is less clear in SCI persons. Waist circumference and serum leptin levels are both potential indicators of obesity and increased risk of cardiovascular disease in the SCI population.4,5 Fat mass is often markedly higher in SCI persons; one study noted a fat mass 8–18% higher than that of age-, height-, and weight-matched controls.4 Many SCI persons with normal BMI would be considered obese if percent body fat were measured;3 this would markedly increase the obesity prevalence in the SCI population.
During the initial weeks after SCI, patients tend to lose weight due to hypercatabolism.6 Paralyzed muscle atrophies, which contributes to decreased basal metabolic rate (BMR). Reduced fat-free mass, sympathetic blunting, cardiopulmonary dysfunction, work capacity reductions, and diminished anabolic hormones sustain the BMR decrement after the acute phase of SCI.3 Energy requirements for physical activity also decline due to relatively sedentary lifestyles. Without diet adjustment to new metabolic requirements after SCI, energy intake quickly exceeds energy requirements, resulting in weight gain.1 Based on clinical observations, Cox et al.7 suggest that SCI patients tend to become obese during the initial 12 months after SCI. However, no systematic study has been performed to document the temporal course of weight gain following SCI.
Obesity is associated with cardiovascular risk factors including dyslipidemia, hyperinsulinemia, glucose intolerance, and hypertension. Cardiovascular disease is a major cause of morbidity in SCI persons, particularly if SCI duration exceeds 10 years.4 In addition to increased tendency toward obesity, SCI persons also have other cardiovascular risk factors. High-density lipoprotein cholesterol levels are 20–42% lower and triglyceride levels are 6–60% higher.4 Impaired glucose tolerance, insulin resistance, and diabetes are also more common in SCI individuals.4 These metabolic changes are partly due to the increased fat mass and loss of fat-free mass.8
SCI persons are at special risk for other obesity-related complications, including sleep apnea,1 social stigmatization,6 pulmonary emboli, and less function than predicted by the SCI lesion, and pain.9 Obesity poses functional limitations, and restricts physical activity, independence, and community integration.6 Increased body weight affects ease of transferring, joint function, and fracture risk.10 In a study examining functional changes in long-term SCI, Gerhart et al.11 found that 39% of SCI persons reported that weight gain compromised activities of daily living.
Maintaining an appropriate body weight is essential in optimizing both health and function of SCI persons. This study seeks to determine the time period when SCI patients are at greatest risk for weight gain, and to identify predictors of obesity in this population. By better understanding the temporal course of weight gain, we may be better able to provide interventions to prevent SCI obesity.
Methods
After approval from the institutional review board, the charts of all individuals who had been consecutively admitted to the acute rehabilitation program at the Veterans Affairs Puget Sound Health Care System (VAPSHCS) SCI Unit from January 1998 to July 2006 were retrospectively reviewed. Participants included all persons with new SCI completing acute rehabilitation at the center during the study period; those with limb loss, co-morbidities potentially affecting body weight, absence of documented follow-up body weights, and all recorded weights less than ideal body weight were excluded. Body weights were recorded with vital signs at intervals closely corresponding to 1 year when participants presented for routine annual evaluations, and heights were self-reported. Main outcome measures included mean change in BMI between rehabilitation admission and final follow-up, time interval of greatest change in BMI, and distribution of participants according to conventional BMI classification: underweight (BMI < 18.5 kg/m2), low normal (BMI 18.6–22.9 kg/m2), high normal (BMI 23.0–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), obese (BMI 30.0–39.9 kg/m2), and extreme obese (BMI > 40.0 kg/m2). Normal weight classification was split into two groups, low normal and high normal reflecting authors' agreement with Rajan et al.12 that the cutoff for overweight be shifted downward to BMI ≥ 23 kg/m2 in persons with SCI. We also determined associations between those BMI changes and SCI level, American Spinal Injury Association Impairment Scale (AIS) grade, primary mode of indoor and community mobility as self-reported during physical and occupational therapy portions of annual evaluations, and age at time of admission to acute rehabilitation.
Statistical significance of the change in mean BMI between rehabilitation admission and final follow-up was determined using a nonparametric method (Wilcoxon signed-rank test). The association of BMI change with participants' characteristics (SCI level, AIS grades, mode of mobility, age, and initial BMI) was examined with the chi-square test. A probability of less than 5% was considered to be statistically significant.
Results
There were 164 participants admitted to the acute rehabilitation program at the VAPSHCS SCI Unit from January 1998 to July 2006. Eighty five of these were included, and 79 were excluded. Characteristics of those included and excluded are presented in Table 1. The most common reason for exclusion was absence of documented follow-up body weights (n = 46) followed by death within 2 years of rehabilitation admission (n = 18).
Table 1.
Characteristics of persons included and excluded
| Characteristics | Participant | Excluded | P-value |
|---|---|---|---|
| Number (%) | 85 | 79 | |
| Age at admission | |||
| Mean | 47 | 54 | 0.007 |
| Median | 51 | 55 | |
| Range | 19–87 | 18–81 | |
| Gender | |||
| Men | 84 (99) | 77 (97) | 0.3142 |
| Women | 1 (1) | 2 (3) | |
| Mechanism of injury | |||
| Traumatic | 57 (67) | 46 (58) | 0.0937 |
| Nontraumatic | 28 (33) | 33 (42) | |
| Neurological level of injury | |||
| Tetraplegia | 55 (65) | 48 (61) | 0.4804 |
| Paraplegia | 30 (35) | 31 (39) | |
| AIS grade | |||
| A | 26 (31) | 21 (26) | 0.0002 |
| B | 6 (7) | 11 (14) | |
| C | 12 (14) | 22 (28) | |
| D | 41 (48) | 25 (32) | |
| BMI (kg/m2) classification at admission | |||
| Underweight (<18.5) | 3 (4) | ||
| Low normal (18.5–22.9) | 28 (33) | ||
| High normal (23.0–24.9) | 14 (16) | ||
| Overweight (25–29.9) | 24 (28) | ||
| Obese (30.0–39.9) | 14 (16) | ||
| Extreme obese (>40.0) | 2 (2) |
All statistical analyses were performed with chi-square test with Yates' correction except for age, which was calculated using paired t-test.
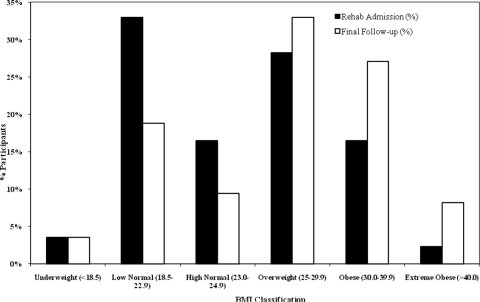
Across all included subjects mean BMI increased by 2.3 kg/m2 (P < 0.001) between rehabilitation admission (mean 45 days post-injury) and final follow-up (mean 5 years post-injury). There was a shift in the distribution of participants from lower BMI classifications (underweight, low normal, and high normal) at time of rehabilitation admission to higher BMI classifications (overweight, obese, and extreme obese) at the time of final follow-up (Fig. 1). Using BMI to define overweight and obese, 47% (40 of 85) of participants were initially overweight or obese but by final follow-up this was 68% (58 of 85).
Figure 1.
Percentage of participants in various BMI classifications at rehabilitation admission versus final follow-up.
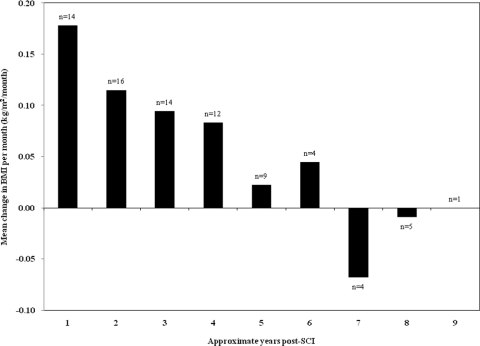
Of those participants transitioning from BMI in the normal range to BMI in the overweight or obese range (n = 22), the greatest increase occurred during the first follow-up interval, which closely corresponded to 1 year after acute rehabilitation admission (Fig. 2). Of the participants with BMI increases >2.0 kg/m2 between initial rehabilitation and final follow-up, the mean change in BMI was 2.8 kg/m2 during the first year post-SCI, 1.1 kg/m2 during the second year, and 0.1 kg/m2 during the third year.
Figure 2.
Mean change in BMI per month for participants transitioning from normal to overweight/obese. Years post-SCI approximates the 1–3 years date post-SCI.
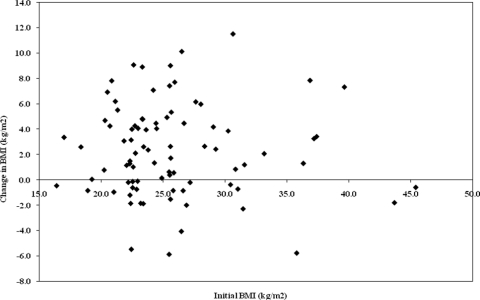
SCI level (tetraplegia versus paraplegia), AIS grade (A–C versus D), mode of both indoor and community mobility, and age at time of acute rehabilitation admission were not significant factors in predicting change in body weight (P ≥ 0.845 for all characteristics). BMI at time of rehabilitation admission was significantly correlated to BMI at final follow-up interval (P < 0.0001). However, the amount of weight gain did not correlate with initial BMI (Fig. 3). Participants that were normal weight during initial rehabilitation were still at risk for significant weight gain.
Figure 3.
Change in BMI versus initial BMI.
Discussion
Obesity is a major health issue in the US in both able-bodied and disabled populations. Previous studies have shown that two-thirds of SCI US military veterans are overweight or obese.1,3 In our study, the prevalence of overweight and obesity at final follow-up matches rates in these previous reports of spinal cord injured US veterans with 68% of participants being either overweight or obese according to BMI. This figure climbs to 78% after shifting the cutoff for overweight downward to BMI ≥ 23 kg/m2, as has been suggested to reflect the loss of fat-free mass (muscle, bone) in SCI persons and consequent under-estimation of obesity by BMI.12
This pilot study provides initial evidence of major weight gain during the first year after SCI. For participants transitioning from normal BMI to overweight or obese, the mean change in BMI per month was 0.18 kg/m2/month for the first year post-SCI, compared to less than 0.11 kg/m2/month for all other follow-up periods. This rate of BMI gain for the first year corresponds to about 6.8 kg or 15.0 lb weight gain, for the average height male. For participants with BMI increases >2.0 kg/m2 between initial rehabilitation and final follow-up, the mean BMI increase during the first follow-up period was 2.8 kg/m2 compared to 1.1 kg/m2 during the second follow-up period and a slight decline during the third follow-up period. In 1985, Cox et al.7 anticipated our finding; they stated ‘It has been our subjective impression that our spinally injured patients have consistently gained weight and become obese … over 12 months, after an acute post injury weight loss’. In acute SCI patients undergoing rehabilitation, they described a remarkably rapid rate of weight gain of 1.3–1.8 kg per week for spinally injured participants after initial weight loss of 5.3–9.1 kg; they did not find a significant difference in weight gain and loss comparing persons with paraplegia versus tetraplegia.7 Our findings and those of Cox et al. indicate that the transition to obesity often occurs in the first year after SCI; seemingly, patients are unable to adequately adjust caloric intake downward to match their decreased metabolic caloric need. These findings emphasize that more aggressive weight gain prevention measures may be required during acute SCI rehabilitation and during the first 6–12 months after SCI.
In this study, the SCI level (tetraplegia versus paraplegia) was not predictive of significant weight gain, measured as BMI gain. In contrast, a higher prevalence of obesity was found in paraplegic versus tetraplegic persons (33 versus 28%), in a study of US military veterans by Gupta et al.1 They suggested that persons with paraplegia have full use of their upper limbs, consequently, allowing them to eat freely.1 Rajan et al.12 proposed an alternative explanation: BMI measures total body weight, and lean body tissues have higher density than adipose tissue. Thus, persons with paraplegia may have a higher percent of lean body mass than those with tetraplegia, and BMI may underestimate obesity more for individuals with tetraplegia than paraplegia. The differences in findings between our study and the one by Gupta et al. may also reflect a difference in participant populations, with relatively new SCI in our study versus long-standing SCI (mean SCI duration 19 years) in the Gupta et al. study.
Another variable not predictive of weight gain was AIS grade. This was surprising as it was anticipated that those with more complete injuries would have greater mobility and exercise limitations, and therefore, be more likely to become obese. In future studies, ASIA motor scores may be a better proxy for mobility and exercise potential than the SCI level (tetraplegia versus paraplegia) and AIS grade (A–C or D).
BMI at time of rehabilitation admission was significantly related to BMI at final follow-up interval, but the amount of weight gain did not appear to depend on initial BMI. Those that were overweight or obese at the start were likely to remain overweight or obese, but they were not likely to gain more weight than were participants that started at normal weight.
In our view, the clinical implication of this study is that weight management needs special emphasis during SCI initial rehabilitation and during the first year post-SCI. Physicians may not adequately prescribe diet and exercise to their disabled patients, perhaps because of perceived hindrances to exercise. However, exercise can contribute to weight loss in SCI persons.2,13 Chen et al.6 demonstrated that intensive behavioral intervention with classes in nutrition and exercise can achieve weight loss and fat mass reduction in chronic SCI persons. The group conducted a pilot study assessing effectiveness of a weight loss program in SCI individuals. Using a diet that emphasized high-bulk low-energy-density foods (e.g. vegetables, fruits, high-fiber grains, and cereals) and reduced high-energy-density foods (meats, cheeses, sugars, and fats) as well as a once weekly educational session, they showed that weight loss is achievable in overweight SCI persons without compromising muscle mass and overall health. Their cohort, which included both persons with paraplegia and tetraplegia, averaged 3.8% loss of body weight in 12 weeks.6 Perhaps, a similar program for those with new SCI, extended over the first year post-SCI, can prevent or reduce the transition to obesity that often occurs. Such a program will also need to emphasize adequate nutrition, to prevent micronutrient deficiencies that have been documented in chronic SCI persons.14–16
We acknowledge limitations of this study. The retrospective data collection with possible charting or weight measurement inaccuracies, self-reported heights, variable follow-up among participants, and only 85 total participants are limitations. Another limitation was the lack of physical activity records as higher levels of physical activity have been shown to correlate with lower BMIs in the SCI population.17 Women were underrepresented in our study, as it reflects US military veterans with SCI. Participants were younger (median age 51 years) compared to those that were excluded (median age 54 years), but it is unclear how much clinical significance this difference makes. Also, participants differed from those excluded in terms of AIS grades, but it is difficult to discern a pattern to account for those differences. As Laughton et al. demonstrated, current BMI cutoffs neglect to identify obesity in many persons with SCI as defined by the fat mass percentage and risk of obesity-related chronic diseases based on elevated C-reactive protein levels. They recommend lowering BMI cutoffs to 22 kg/m2 to better identify those at high risk for obesity and obesity-related diseases.18 Our use of BMI at time of acute rehabilitation admission when participants likely had greater percentages of lean body mass compared to follow-up measurements when muscle atrophy and bone mineral density loss had increased, likely underestimates the true gain in fat mass.
Use of dual-energy X-ray absorptiometry (DEXA) may be a preferred method for quantifying body composition. This method has been shown to identify individuals with body fat percentages consistent with obesity even when BMI is in the normal to overweight range.19 It is more commonly used and has been shown to be more accurate than skin fold thickness measurements and bioelectrical impedance analysis.20 Using DEXA, Spungen et al. demonstrated that total body and regional lean mass were lower and fat mass was higher in persons with SCI compared with controls. They also showed that persons with SCI were significantly fatter per unit of BMI compared with controls.21 In a study of monozygotic twins discordant for SCI, Spungen et al.22 used DEXA to demonstrate that the twins with paraplegia had significantly more fat per unit BMI than their able-bodied co-twins.
Conclusion
These findings confirm significant increase in body weight after new SCI and suggest that the time of greatest weight gain risk for persons with new SCI is during the year following acute rehabilitation admission. This study highlights the need for obesity prevention during initial rehabilitation and the early follow-up period.
Further research aimed at weight gain prevention strategies in the newly injured SCI population is warranted. Examining possibilities for patient education programs during the acute rehabilitation stay may be a vital component of future prevention strategies.
References
- 1.Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury – a retrospective study. Spinal Cord 2006;44(2):92–4 [DOI] [PubMed] [Google Scholar]
- 2.Weil E, Wachterman M, McCarthy EP, Davis RB, O'Day B, Iezzoni LI, et al. Obesity among adults with disabling conditions. JAMA 2002;288(10):1265–8 [DOI] [PubMed] [Google Scholar]
- 3.Gater DR. Obesity after spinal cord injury. Physical Medicine and Rehabilitation Clinics of North America 2007;18(2):333–51 [DOI] [PubMed] [Google Scholar]
- 4.Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord 2005;43(9);513–8 [DOI] [PubMed] [Google Scholar]
- 5.Maimoun L, Puech AM, Manetta J, Badiou S, Paris F, Ohanna F, et al. Circulating leptin concentrations can be used as a surrogate marker of fat mass in acute spinal cord injury patients. Metabolism 2004;53(8):989–94 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Henson S, Jackson AB, Richards JS. Obesity intervention in persons with spinal cord injury. Spinal Cord 2006;44(2):82–91 [DOI] [PubMed] [Google Scholar]
- 7.Cox SA, Weiss SM, Posuniak EA, Worthington P, Prioleau M, Heffley G. Energy expenditure after spinal cord injury: an evaluation of stable rehabilitating patients. J Trauma 1985;25(5):419–23 [PubMed] [Google Scholar]
- 8.Jones LM, Legge M, Goulding A. Factor analysis of the metabolic syndrome in spinal cord-injured men. Metabolism 2004;53(10):1372–7 [DOI] [PubMed] [Google Scholar]
- 9.Buchholz AC, Pencharz PB. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care 2004;7:635–9 [DOI] [PubMed] [Google Scholar]
- 10.McColl MA, Skinner HA. Spinal cord injury and lifestyle health risks. Can J Rehab 1996;9(2):69–82 [Google Scholar]
- 11.Gerhart KA, Bergstom E, Charlifue SW, Menter RR, Whiteneck GG. Long-term spinal cord injury: functional changes over time. Arch Phys Med Rehab 1993;74(10):1030–4 [DOI] [PubMed] [Google Scholar]
- 12.Rajan S, McNeely MJ, Warms C, Goldstein B. Clinical assessment and management of obesity in individuals with spinal cord injury: a review. J Spinal Cord Med 2008;31(4):361–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloemen-Vrencken JH, de Witte LP, Post MW, van den Heuvel WJ. Health behaviour of persons with spinal cord injury. Spinal Cord 2007;45(3):243–9 [DOI] [PubMed] [Google Scholar]
- 14.Walters JL, Buchholz AC, Martin Ginis KA. Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord 2009;47(4):318–22 [DOI] [PubMed] [Google Scholar]
- 15.Tomey KM, Chen DM, Wang X, Brauschweig CL. Dietary intake and nutritional status of urban community-dwelling men with paraplegia. Arch Phys Med Rehab 2005;86(4):664–71 [DOI] [PubMed] [Google Scholar]
- 16.Moussavi RM, Garza HM, Eisele SG, Rodriguez G, Rintala DH. Serum levels of vitamins A, C, and E in persons with chronic spinal cord injury living in the community. Arch Phys Med Rehab 2003;84(7):1061–7 [DOI] [PubMed] [Google Scholar]
- 17.Buchholz AC, Martin Ginis KA, Bray SR, Craven BC, Hicks AL, Hayes KC, et al. Greater daily leisure time physical activity is associated with lower chronic disease risk in adults with spinal cord injury. Appl Physiol Nutr Metab 2009;34(4):640–7 [DOI] [PubMed] [Google Scholar]
- 18.Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE. Lowering body mass index cutoffs better identifies obese person with spinal cord injury. Spinal Cord 2009;47(10):757–62 [DOI] [PubMed] [Google Scholar]
- 19.Jones LM, Goulding A, Gerrard DF. DEXA: a practical and accurate tool to demonstrate total and regional bone loss, lean tissue loss and fat mass gain in paraplegia. Spinal Cord 1998;36(9):637–40 [DOI] [PubMed] [Google Scholar]
- 20.Mojtahedi MC, Valentine RJ, Evans EM. Body composition assessment in athletes with spinal cord injury: comparison of field methods with dual energy X-ray absorptiometry. Spinal Cord 2009;47(9):698–704 [DOI] [PubMed] [Google Scholar]
- 21.Spungen AM, Adkins RH, Steward CA, Wang J, Pierson RN, Jr, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407 [DOI] [PubMed] [Google Scholar]
- 22.Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft issue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 2000;88(4):1310–5 [DOI] [PubMed] [Google Scholar]