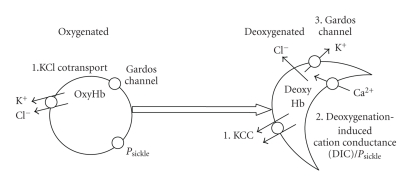
Figure 1.
Schematic diagram of the main transport pathways activated in red blood cells (RBCs) from sickle cell patients. In RBCs from homozygous HbSS individuals, high cation permeability is accounted for by three main pathways [28, 37]. Under oxygenated conditions, the KCl cotransporter (KCC) is highly active. It is overexpressed in HbSS cells compared to HbAA ones and does not become quiescent as RBCs mature. It is stimulated further by low pH (reduction in extracelluar pH from 7.4 to 7). Under deoxygenated conditions, KCC remains active—again unlike the situation in HbAA RBCs [40]. In addition, two other pathways are observed. The deoxygenation-induced cation conductance (or P sickle) is activated as HbS polymerises. It mediates entry of Ca2+. Elevation in intracellular Ca2+ then leads to activation of the third pathway, the Ca2+-activated K+ channel, or Gardos channel. These three pathways result in solute loss, cell shrinkage and dehydration, and consequent increase in [HbS]. They thereby contribute to pathogenesis of sickle cell disease. They are also likely to be involved in solute loss from RBCs of patients heterozygous for HbS and HbC (HbSC genotype), though details are lacking and differences in their behaviour compared to that in HbSS cells are expected.